How Does Temperature Affect Selectivity?
Column temperature plays an important role in controlling peak spacing (selectivity) in reversed-phase liquid chromatography (LC) separations. Temperature has long been known to affect retention time, and more recently, its use in adjusting selectivity has gained popularity (see reference 1 for a review of temperature selectivity). In preparation of a paper for the most recent Pittsburgh Conference, I had an opportunity to reexamine some data that compare temperature selectivity with other variables used to control selectivity in LC separation. This month's instalment of "LC Troubleshooting" examines temperature selectivity and its relationship to pH selectivity.
Column temperature plays an important role in controlling peak spacing (selectivity) in reversed-phase liquid chromatography (LC) separations. Temperature has long been known to affect retention time, and more recently, its use in adjusting selectivity has gained popularity (see reference 1 for a review of temperature selectivity). In preparation of a paper for the most recent Pittsburgh Conference,2 I had an opportunity to reexamine some data that compare temperature selectivity with other variables used to control selectivity in LC separation. This month's instalment of "LC Troubleshooting" examines temperature selectivity and its relationship to pH selectivity.
Selectivity and Temperature
One common way to plot changes in peak spacing with a change in a variable is to use a resolution map. This is illustrated in Figure 1(a) for the effect of temperature on resolution for a mixture of 11 weak acids and bases. The resolution map plots the resolution for the least-resolved pair of peaks in the separation for different column temperatures. The best resolution is seen for the high points on the plot, at approximately 34 °C and 41 °C, whereas two peaks overlap completely whenever the plot dips to the zero resolution baseline. The resolution at 34 °C is illustrated in Figure 2(a), where all 11 peaks are resolved from each other. Moving to lower temperatures causes peaks 5 and 6 (4-n-butyl benzoic acid and 4-n-hexyl aniline) to move together; higher temperatures cause peaks 3 and 4 (4-n-pentyl aniline and diflunisal) to move together — 34 °C is the balance point where both peak pairs have the same resolution. In a similar manner, the resolution at 41 °C [Figure 3(a)] between peaks 6 and 7 (4-n-hexyl aniline and diclofenac acid) is balanced with that of peaks 1 and 2 (2-phenyl pyridine and ketoprofen).
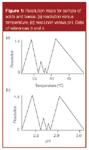
Figure 1
Resolution maps are powerful tools for the quick identification of resolution maxima and can be useful to help estimate method robustness to changes in the variable under consideration. Such maps are key features of resolution modelling software, such as DryLab (Molnar Institute, Berlin, Germany). Resolution maps that have many peak reversals (dips to zero resolution), such as the one in Figure 1(a), are more common with samples containing ionic compounds than for neutral compounds.
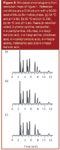
Figure 2
Selectivity and pH
When I examined the resolution map for temperature selectivity for the mixture of acids and bases used here, it struck me that it looked very much like resolution maps that I see for pH as the variable. So I plotted a map of resolution versus pH for the same sample, based upon the data of references 3 and 4. In Figure 1(b), I have shown just a portion of the pH map normalized to approximately the same layout as the section of the temperature map of Figure 1(a). They are strikingly similar. They have the same peaks forming the least resolved pairs in each region of the resolution map and the overall maximum resolution is approximately the same in both instances. So just as we had maxima at 34 °C and 41 °C in Figure 1(a), we have corresponding maxima at pH 2.785 and 2.910 in Figure 1(b). Compare the chromatograms for these temperature and pH conditions in Figures 2(a) and 2(b) (34 °C versus pH 2.785) and 3(a) and 3(b) (41 °C versus pH 2.910). For each pair of chromatograms, the retention order is identical and the peak spacing is almost the same. Also note that the higher temperature of the 41 °C run reduced the retention times of all the peaks, as expected for an increase in temperature.

Figure 3
But Is It Real?
Resolution maps represent predicted separations based upon input data from two (or more) "calibration" runs. In the present instance, the temperature map was based upon a run at 35 °C and one at 45 °C, with both runs at pH 2.80. The pH map was made at pH 2.80 and 3.00 for 35 °C. You might suspect that once one interpolates or extrapolates new separations at points other than the input data points, errors are probable. Yes, this is true, and the "garbage-in-garbage-out" axiom of computer programs applies here, as well. However, in my experience, if the data are gathered under carefully controlled conditions (these were), the predictive accuracy is in the ±1–5% region. The comparative results of Figures 2(a), 2(b), 3(a) and 3(b) certainly support this. But these are predicted separations and you say, "Show me the beef" for real runs.

Figure 4
We can compare the results of real experiments by comparing the input runs used to generate the resolution maps of Figure 1. In both instances, the input data were suboptimal on the right side of the two resolution maxima in Figures 1(a) and 1(b). Both maps used a common point of 35 °C and pH 2.80, as shown in
Figure 2(c). A 45 °C, pH 2.80 run was used as a second point to generate the resolution map of Figure 1(a). Similarly, a 35 °C, pH 3.00 run was used for the second point of the resolution map of Figure 1(b). These two runs are shown in Figures 3(c) and 3(d), respectively. They are not a perfect match. First, we notice that the last peak of Figure 3(c) is eluted at approximately 10 min, whereas the last peak of Figure 3(d) is closer to 12 min. Remember that the rule of thumb for a change in retention with temperature says that a 1 °C increase in temperature will decrease retention by approximately 2%. So a 10 °C increase in temperature reduced the retention time by approximately 20%, as expected. Second, note that the first two peaks in Figure 3(c) can be distinguished as a doublet, whereas they are merged into a single peak in Figure 3(d). This agrees with the resolution map data, too — note that the 45 °C point in Figure 1(a) is about halfway up the side of the resolution plot with a maximum at 41 °C, whereas the pH 3.00 point is near the bottom of the corresponding plot of Figure 1(b).
So What's Happening?
One more way to look at the data is shown in Figure 4. Figure 4(a) is the reference run at 35 °C and pH 2.80 [same as Figure 2(c)]. When the temperature was increased to 45 °C, the run of Figure 4(b) resulted [same as Figure 3(c)]. Note that the dotted lines indicate that there were three peak reversals that occurred with the temperature change. Figure 4(c) shows the results for a change in pH from 2.80 to 3.00, both at 35 °C [same as Figure 3(d)]. The dotted lines show that the peak order for the 45 °C and pH 3.00 runs is identical. That is, the exact same crossovers occurred when going from 35 to 45 °C as when changing from pH 2.80 to 3.00.
These data strongly suggest that a change in column temperature has exactly the same effect as a change in pH (at least for this sample). If we think about this, though, it is not too surprising — we know that buffer pH changes with temperature, that sample ionization changes with temperature, and that the ionization of the silanols on the column changes with temperature. Some combination of these changes is probable in the present instance.
However, these results are more than a novelty — they have very practical implications. Note that it takes a 10 °C change in temperature to make the same change as a 0.2 unit change in pH. If you use the pH adjustment technique of titrating the buffer to a desired pH with a pH meter, the normal variation of the actual pH is in the range of ±0.1 pH unit. It is much easier to control the temperature within a degree or two than it is to control the pH within <0.1 pH units. Furthermore, we can "tweak" the temperature a degree or two to adjust a separation much easier than adjusting the pH of the buffer.
What are the take-home lessons? First, temperature is a powerful variable to use to adjust selectivity when ionic compounds are present, so it should be considered when developing a method. Second, because temperature is such a powerful variable, it is very important to use temperature control for your column and to use a buffered mobile phase if you want to obtain reproducible separations with ionic compounds. Finally, a small 1–2 °C change in temperature might be sufficient to make minor adjustments to the method to enhance the separation of a pair of peaks when a small error in buffer preparation was made or column aging causes a reduction in resolution.

A Request for Readers
"LC Troubleshooting" editor John W. Dolan is vice-president of LC Resources, Walnut Creek, California, USA; and a member of the Editorial Advisory Board of LCGC Europe. Direct correspondence about this column to "LC Troubleshooting", LCGC Europe, Advanstar House, Park West, Sealand Road, Chester CH1 4RN, UK.
Readers can also direct questions to the Chromatography Forum at www.chromforum.com
References
1. J.W. Dolan, J. Chromatogr. A, 965, 195–205 (2002).
2. J.W. Dolan, Pittcon 2007, paper 890-2.
3. N.S. Wilson et al., J. Chromatogr. A, 961, 171–193 (2002).
4. N.S. Wilson et al., J. Chromatogr. A, 961, 195–215 (2002).

University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













