How To Buy Gas Calibration Mixes
LCGC Europe
In January 2004, LCGC Europe published an article on methods for selecting pure gases for analytical applications. 1 Here, the authors expand on this topic by addressing questions regarding gas mixtures - defined simply as more than one gas in a cylinder.
There is a great deal of confusion floating about when it comes to selecting gas mixtures for analytical applications. Questions such as "How is the gas certified?" "What standard should I use?" and "What level of uncertainty is acceptable?" are common concerns heard among those tasked with specifying and using mixtures. In addition, the uncertainty regarding terminology, measurement, safety and storage can make specifying mixtures a true source of headaches and hassles for any laboratory manager. The following article provides answers to questions about gas mixtures.
Gas Mixtures
Gas mixtures are used in a host of analytical applications. The most basic mixtures provide specific performance functions, much like pure gases in analytical applications. For example, "instrument air," a synthetic blend of nitrogen and oxygen mixed to concentrations similar to breathing air, supports hydrogen combustion in a flame ionization detector and acetylene combustion in an atomic absorption (AA) flame. Other mixtures, such as those used in a chemical or refinery pilot plant, can be highly complex blends used to mimic a process stream.
However, the most common application for a gas mixture is for calibrating equipment used to monitor processes or emissions. Many of these are gas chromatography (GC) or spectroscopy applications that require calibration standards. Gaseous standards can be simple, such as a blend of oxygen and nitrogen, or can be a complex, multicomponent mixture of hydrocarbons and volatile organic compounds. In selecting these mixtures, a number of issues must be considered to ensure proper calibration.
Selecting the Right Mixture: When it comes to specifying gases for analytical equipment, many cylinder gas users specify a variety of names, such as "primary standard," "master grade," or "certified." But what do these names mean? As with pure gases, there are no universal standards in the gas industry for naming gas mixtures or grades. What one company calls a primary standard can be radically different from what another company calls it.
The exceptions to this rule are medical-grade mixtures used in a variety of applications and Environmental Protection Agency (EPA) Protocol mixtures, which are standards of smog and acid rain–causing chemicals used for environmental monitoring. Mixtures such as these must be certified in accordance with specific procedures outlined by the Food and Drug Administration (FDA), EPA or other regulatory bodies.
The process for certifying EPA Protocols is strictly regulated. The EPA conducts periodic audits of EPA Protocol mixture producers. In its most recent audit, the EPA concluded that all the companies audited have the ability to produce accurate EPA Protocol mixtures and that its data should not be used to rank vendors. Despite this, some gas producers have attempted to rank the vendors. In reality, most of the major EPA protocol producers supply compliant product, and one vendor's mixtures will not provide significant performance advantages over another.
For most applications, however, specifying the right gas depends upon understanding how mixtures are qualified. It is important to remember that as a general rule, the level of uncertainty and mixture complexity will dictate the cost. Therefore, it is advisable to choose a gas that meets the minimum requirements of the application to avoid overspending on gas purchases. This next section highlights the most important factors that go into specifying gas mixtures.
Tolerance: The precision and accuracy of the mixture need to be defined when making a specification. Again, the lack of universal standards makes it difficult to rely on the name or grade. A better method for making a selection is to understand the application and acceptable tolerance associated with the particular mixture in question.
There are two types of tolerance or categories of error that gas companies should specify when naming a standard: blend or preparation tolerance and analytical or certification tolerance.
The preparation tolerance or allowable deviation from the requested component concentration is a result of the error associated with mixing the gas components. Gas mixing contains inherent inaccuracies because of the equipment used in production and the human element that is part of most filling applications. The analytical tolerance is the minimum acceptable uncertainty at a defined confidence level associated with the analysis of the blend. This uncertainty is accumulated throughout the analytical process and includes instrument and calibration uncertainties.
For most applications, the analytical tolerance is of greater importance than the preparation tolerance because it represents the actual concentration range of the analysed component. The preparation tolerance becomes equally if not more important only in applications that require an upper or lower range of concentration that cannot be exceeded. For example, a certified mix ordered at 100 ppm with a ±10% preparation tolerance is prepared between 90 ppm and 110 ppm. Assume the mix reads 105 ppm when it is made. When analysed in the laboratory with a ±2% certification tolerance, it might actually be between 103 ppm and 107 ppm (see Figure 1).
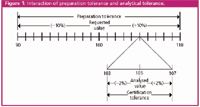
Figure 1: Interaction of preparation tolerance and analytical tolerance.
Accuracy versus precision: In discussing mixtures, the terms precision and accuracy are often used incorrectly. These terms are tied to preparation and analytical tolerance and should not be used interchangeably. Precision, also called consistency, refers to the blending of the mix, whereas accuracy, also called uncertainty, is used when discussing the analytical values of the minor components (see Figure 2).
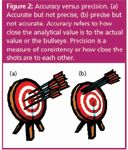
Figure 2: Accuracy versus precision. (a) Accurate but not precise, (b) precise but not accurate. Accuracy refers to how close the analytical value is to the actual value or the bullseye. Precision is a measure of consistency or how close the shots are to each other.
There are many factors that determine how precise a mix can be made. The concentration of the minor components in the gas significantly impacts the precision. The lower the levels, the more difficult it is to blend the mix. Another consideration is the method of blending used to make the gas. For example, blending by pressure and volume, also known as partial pressure or manometric blending, is the least precise manufacturing technique. For most low-level mixtures, gravimetric blending or mixing by weight is the most accurate method.
Even gravimetric blending does contain some degree of error, as there are measurement uncertainties associated with the performance of the scale as well as a human element involved. An inexperienced blender might have a difficult time blending low-level mixes within the preparation tolerance. To resolve this problem, speciality gas companies have developed fully automated gravimetric blending systems that provide very precise mixes on a highly consistent basis (see Figure 3). This can be particularly important for certain gas mixtures, such as those used to precisely mimic a process stream.

Figure 3: Automated gravimetric blending: Producing a precision mixture using automated gravimetric blending (monitoring the process is Rob Shock of Airgas, Radnor, Pennsylvania, USA).
Traceability: A requirement for ISO 9001:2000 programmes, ISO 17025 compliant programmes, emissions monitoring and reportable environmental testing is that the instrument calibration process maintain traceability to a national primary reference material. This is usually verified by one of two methods:
- Analytical traceability: Using reference materials such as Standard Reference Materials (SRMs) and National Institute of Standards and Technology (NIST) Traceable Reference Materials (NTRMs) from a national measurement institute (usually NIST) to calibrate the measurement system through a rigorous process to determine the concentrations of mixture components of interest.
- Process traceability to the international unit of mass, the kilogram, through comprehensive manufacturing and quality programmes, using high-precision, high-sensitivity weighing systems for component additions.
The resultant mixtures are analysed against laboratory primary standards of known composition and uncertainty. Typically, this process-based traceability is used when reference materials are unavailable from NIST or other national measurement institutes for the component(s) or concentration(s) of interest. Blends produced gravimetrically, using scales calibrated with NIST certified weights, are considered traceable and have known uncertainty in their composition.
It is interesting to note that in the automotive industry, there is an added requirement to use standards directly traceable through analysis to the NIST. Working with suppliers who offer ISO 17025–accredited facilities can provide an extra degree of credibility, as this accreditation provides validation of analytical techniques, methodology and competency.
Once the level of acceptable uncertainty and traceability have been specified, a host of other factors must be considered to ensure proper mix selection.
Units of measure: When a cylinder is analysed, the concentration levels of the mixture should be listed with the appropriate unit of measure. Levels typically are listed as mole percent, volume percent or weight percent. The listed amounts will differ depending on the unit of measure (see Table 1).
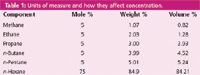
Table 1: Units of measure and how they affect concentration.
It is essential to make sure that specifications are evaluated in identical units to limit bias in your evaluation. A useful analogy to consider is a house being built by two carpenters. One uses English units, while the other works in the metric system. Before long, the floor would tilt and the walls would cave in. Similarly, it is important for vendors to "talk" in the same units as the analyst.
Mixture safety: With all mixtures, the most important item to consider is whether the mixture can be blended safely. This is a key concept, as there are many mixtures in which the final product is safe, but the blending procedure carries some risk. For example, concentrations of flammables and oxidants, such as 2.5% methane in air, are especially complicated to manufacture safely. It is vital that strict procedures are followed to ensure that the Btu value of the mixture meets industry safety standards both in the manufacture and use of the gas mixture.
There are three factors that control the blending of these mixtures: fuel, the presence of an oxidizer and a source of ignition. To prevent the mixture from becoming flammable, the concentration of fuel in the mixture is limited, making the mixture "fuel lean." Another way the release of energy can be limited is by controlling the oxygen concentration. Without an oxidant, the fuel will not burn. A safe filling method is to assume that a source of ignition is present, so that the lower explosive limit of the gas and the minimum oxygen for combustion are not exceeded.
Mixture stability: Mixture stability refers to how stable the individual components will be over time. Will they remain stable or will they begin to react? To assess this, the component compatibility and reactivity must be factored into gas mixture manufacture and storage. For example, components like nitric oxide (NO) will react in the presence of trace amounts of oxygen to form nitrogen dioxide (NO2). This reaction can cause the NO concentration to degrade over time. Conversely, without the presence of oxygen in a mixture containing NO2, there can be conversion to NO. Therefore, proper precautions should be taken by the gas manufacturer to ensure stability.
Absorption or reaction of components with the cylinder walls can also impact the mixture's stability. To control this, it is important that the package be designed specifically for the gases it will contain. In some instances, this can mean using an aluminium cylinder instead of a steel cylinder. Another method that can increase stability is "passivating" the cylinder before filling, a common technique with reactive component blends. This process involves introducing a blend of the reactive component or other passivating material into the cylinder before filling with the end product.
Raw material specification: It is important to understand the impact of contaminants in the raw materials of a mixture on an operation. As with pure gases, the overall purity of the gas is less important than the actual nature of impurities in the gas.1 Critical contaminants such as hydrocarbons, oxygen or moisture can affect calibration, create reactivity of mixture components, or cause other analytical problems.
In many analytical applications, an initial raw material check is all that is required, although it is possible that an application might require a final check by the vendor to ensure proper specification. In the case of multicomponent hydrocarbon blends, gas manufacturers should analyse the incoming raw materials and document the individual hydrocarbon contaminants. If the gas company does not account for all possible hydrocarbons in the raw material, then the mixture can be out of specification when analysed. Also, what is a contaminant in one hydrocarbon product actually might be a separate component in the calibration mixture. For example, ethane is a common contaminant in ethylene. If a calibration standard is required that contains both hydrocarbons, the ethane in the ethylene raw material must be considered to ensure an acceptable ethane level.
Shelf-life: Shelf-life is an estimation of how long the mixture components are expected to remain within the certification tolerance after the mixture's certification date. For most inert components such as nitrogen, argon and carbon dioxide, the shelf life is estimated in years. For example, mixtures of oxygen and nitrogen will remain stable indefinitely. However, reactive components such as certain oxides, sulphur compounds and polar compounds might last only months when mixed. In general, shelf life is not specified, except in the case of EPA Protocol and medical mixtures in which expiration dates are required. Vendors should specify a mixture shelf life if reactivity of components is a concern.
Phase: Phase can affect the concentration of components in the mixture. Mixtures of gases used to calibrate instrumentation might be in either gas or liquid phase depending upon the physical characteristics of the components in the mixture. This can impact the concentration of the various components.
When a component in a high-pressure mixture is cooled below its dew point, it condenses. Once this happens, the mixture is compromised and the condensed component will read lower than the certified amount. For this reason, gaseous mixtures that contain components that condense at high pressures or low temperatures must be prepared and stored carefully.
There are some applications where a liquid-phase sample is assayed, requiring that a liquid-phase mixture be used for calibration. Most liquid-phase mixtures contain both a gas phase and a liquid phase, with the components at equilibrium. The concentrations of these components will change as the liquid level in the cylinder drops as product is used, unless kept under a constant, high pressure. Constant pressure can be kept on liquid mixes through use of a "piston" cylinder or inert gas head pressure. Sudden swings in temperature or a rapid withdrawal of the mixture will upset the vapour–liquid phase equilibrium, making a representative sample impossible to obtain. Components with a low boiling point and high vapour pressure will tend to concentrate in the vapour phase. As product is initially withdrawn from the cylinder, vapour phase components will be at their highest levels. Components with a high boiling point and low vapour pressure, such as heavy hydrocarbons, will tend to concentrate in the liquid phase. Therefore, it is important to stipulate the phase that should be analysed and ensure multiple vendors are sampling and analysing consistently.
What's in a Name?
Understanding the correct terminology can help when specifying a gas. Although you might not see these names listed, most manufacturers' gas mixtures tend to fall into one of the following categories:
- EPA protocol standards
- Traceability standards
- Precision standards
- Primary standards
- Certified standards
- Unanalysed mixtures
EPA Protocol standards: EPA protocol gases are manufactured and analytically certified in accordance with the most recent EPA traceability guideline document entitled "EPA Traceability Protocol for Assay and Certification of Gaseous Standards."2 According to this standard, mixtures must be certified to a ±2% overall uncertainty. However, the majority of EPA protocol gas suppliers certify to a ±1% overall uncertainty, except where limited by the higher uncertainty of the NIST SRMs or NTRMs. EPA protocol gases should only be used when required by law.
Traceability standards: Traceability standards are calibration mixtures, which are analytically certified directly against either NIST SRMs or NTRMs. The analytical testing process is based upon the EPA Protocol document, including triad analysis to compensate for short-term instrumental drift, comprehensive instrument characterization and statistical data analysis. Typically, there is a ±1% overall uncertainty with direct traceability to NIST reference materials. A traceability standard should be used in select applications in which direct traceability through analysis is required. In reality, however, most mixes marketed as "directly NIST traceable" are traceable to NIST weights but are not directly traceable to NIST calibration standards. These mixes might not meet requirements of those requiring direct analytical traceability.
Precision blends: Precision blends are developed to satisfy customer requirements for "zero blend tolerance" mixtures. These blends are manufactured by mixing the gas components dynamically and continuously monitoring the composition to account for any drift in component concentration. It is impossible for a vendor to supply a truly "zero tolerance" mixture because of inherent equipment and human error. However, precision blending can produce mixtures that statistically are identical to the requested concentration. Keep in mind that for most calibration applications, "zero" preparation tolerance offers limited advantage, except for those that attempt to mimic a process stream.
Primary standards: Primary standards, which are often referred to as "NIST traceable by weight mixtures," are used when applications demand a high degree of accuracy and reliability. Most gas mixture manufacturers produce and certify primary standards gravimetrically on sophisticated high-load, high-sensitivity scales, because analytical testing introduces a laboratory bias. The method for providing certified values varies from vendor to vendor, with some supplying the analytical value obtained in the laboratory and others supplying the gravimetric scale value. Whatever the method, it is important the supplier use weighing systems that are calibrated stringently with NIST Class S weights.
Certified standards: Certified standards, which are sometimes referred to as "working standards," are analysed calibration mixtures used routinely in science and industry. Certification of the standard is performed through quality control analysis on laboratory instrumentation. For the majority of applications, including instrumentation calibration, the tolerance of a certified standard is acceptable. These standards are generally prepared either by partial pressure, using pressure and temperature as a proxy for component concentration or gravimetrically.
Unanalysed mixtures: Although prepared by the same techniques as certified standards, unanalysed mixtures are not verified or checked by analysis. These mixtures should be used in applications where mixture accuracy is less of an issue.
Conclusion
Understanding the factors that go into making a specification are an important step to making the right selection of a gas calibration mixture. Rather than buying by name, users should understand the requirements of their applications. By specifying tolerance, traceability, accuracy and precision, as well as a host of other requirements ranging from measurement to safety, users can make better choices and achieve the truest results. Additionally, working with a supplier that understands gas mixtures and can provide guidance regarding elements such as cylinder movement, modes of supply, storage and reverse supply chain can simplify the process and let chromatographers focus on the work at hand, rather than thinking about their "gas pains."
Steve Scheuring is Marketing Manager of speciailty gases with Airgas Inc., Radnor, Pennsylvania, USA.
Daniel Bartel is Director of Technical Support Services with Airgas Inc., Chicago, Illinois, USA.
References
1. S. Scheuring, LCGC Eur., 17(1), 16–22 (2004).
2. EPA-600/R97/121, EPA Traceability Protocol for Assay and Certification of Gaseous Calibration Standards.

Study Examines Impact of Zwitterionic Liquid Structures on Volatile Carboxylic Acid Separation in GC
March 28th 2025Iowa State University researchers evaluated imidazolium-based ZILs with sulfonate and triflimide anions to understand the influence of ZILs’ chemical structures on polar analyte separation.











