Green Chemistry Perspectives on Analytical Extractions
Special Issues
This article introduces some of the concepts behind green chemistry, covering solvent selection and extraction techniques.
The growing interest in green chemistry requires fresh perspectives on analytical extractions. Reduced solvent consumption, alternative safer solvents, and reasonable energy demands must be balanced with traditional analytical considerations such as extraction yield and selectively. This article introduces some of the concepts behind green chemistry, and discusses green solvent selection and extraction techniques. An overview of alternatives to conventional solvents, new green solvents, ionic liquids, and other solvent options are also described.
The history of modern analytical extractions mirrors the development of green chemistry. For nearly a century Soxhlet extraction, combined with shake-flask methods, was the standard method for the isolation of analytes from solid samples, while multiple liquid–liquid extractions (LLEs) using separatory funnels was the method of choice for liquid samples. In the mid-1980s, new forms of analytical extractions were developed and popularized — such as supercritical fluid extraction (SFE), pressurized-fluid extraction (PFE), solid-phase extraction (SPE), solid-phase microextraction (SPME), microwave-assisted extraction (MAE), single-drop microextraction (SDME), and ultrasonic extraction. These new extraction techniques had benefits such as faster times, lower cost for each extraction, improved yield and reproducibility, and lower solvent volumes. Lower solvent volumes provide significant environmental advantages. Extraction solvents generally provide the bulk of the waste encountered in any analytical method and often have health and safety concerns, such as toxicity or flammability.
In parallel with these developments, the concept of green chemistry emerged. This culminated in the statement of the 12 principles of green chemistry in 1998, which are shown in Table 1 (1). The goals of green chemistry are to address environmental, health, and safety concerns when planning a chemical process rather than after it has been performed. Despite the fact that modern extraction technologies and green chemistry are contemporaries, the two fields have advanced independently of each other. The green advantages of newer extraction methods are widely promoted, but they are rarely placed in the context of the green chemistry principles.

Table 1: Principles of green chemistry. Adapted from reference (1).
Green chemistry in the chromatography laboratory was recently reviewed and this article described ways to save on solvent consumption, alternatives to using acetonitrile in liquid chromatography (LC), and how to assess the "greenness" of analytical methods (2). These topics will not be repeated in this review.
Green Chemistry in the Planning Stages
A somewhat tongue in cheek, though serious, adage is that "the 'greenest' analytical procedure is that which you do not perform"— but well-executed analytical methods can inherently be green. Following correct sampling theory (as outlined in several undergraduate textbooks), the minimum number of samples and minimum sample size is desired. Samples collected should also support the problem-solving efforts behind the analysis.
One disturbing trend of many modern extraction technologies, that runs counter to the planning of an analysis, is the trend toward miniaturization. Miniaturization is advantageous when used properly, but it seems that many analysts embrace miniaturization because they can, rather than following appropriate sampling theory. Miniaturization may reduce solvent use and waste generation, but it does not address, for example, solvent toxicity.
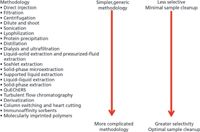
R.E. Majors recently reported on developments in an article entitled '"Just Enough" Sample Preparation: A Proven Trend in Sample Analysis' (3). Following the examples of "just-in-time" inventory control in total quality management systems, "just-enough" sample preparation balances analytical considerations such as selectivity and quantitative needs with labour, time, and solvent intensiveness. Important "just-in-time" methods are presented in Figure 1. This approach reiterates the importance of understanding the purpose of a given analysis:
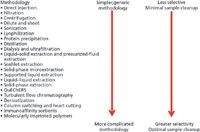
Figure 1: Summary of just-in-time sample preparation methodologies and their attributes (3).
"Minimizing the number of sample handling steps in any analytical technique is desirable since the more times the sample is transferred, the greater the chance of analyte loss (or modification), thereby resulting in poor analytical precision and accuracy," — R.E. Majors (3).
Similarly, the appropriate choice of the subsequent analytical characterization technique can drive sample preparation and the overall "greenness" of an analytical procedure. For example: Desorption electrospray ionization (DESI) and direct analysis in real time (DART) approaches to mass spectrometry (MS) eliminate or minimize the need for sample preparation (4,5,6). In both approaches, the sample (often in solid form) is bombarded by energetic species to create ions of interest. Further development and maturation of these techniques (and those that they have inspired), will contribute to the overall "greenness" of the analytical endeavour.
The use of the biodegradable chelant ethylene diamine disuccinate (EDDS) has been investigated as a substitute for the common ethylene diaminetetraacetic acid (EDTA) in analytical applications (7). Table 2 displays the quantitative results for the isolation of aqueous divalent cations. Although this review focuses on the sample preparation of organics for subsequent chromatographic analysis, these results demonstrate the application of green chemistry principles across a broad spectrum of chemical analysis.
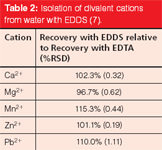
Table 2: Isolation of divalent cations from water with EDDS (7).
Green Sample Preparation Methods
As emphasized, nearly all of the extraction technologies developed within the past twenty years tend to have green advantages, regardless of whether that was the initial intent. SFE, MAE, accelerated-solvent extraction (ASE), and the use of superheated water for analytical extractions are all discussed elsewhere in this supplement. Table 3 shows a comparison of the green attributes of these, and similar, sample preparation methods (8). Here, we present a brief overview of analytical extraction technologies with green attributes.
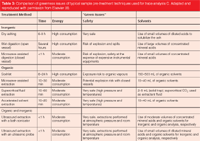
Table 3: Comparison of greenness issues of typical sample pre-treatment techniques used for trace analysis C. Adapted and reproduced with permission from Elsevier (8).
Micro-Liquid–Liquid Extraction: In a review of classical extraction procedures, Murray reported an approach for extracting large liquid sample volumes with a minimal amount of solvent, mixing 980 mL of aqueous sample and 200 μL of hydrocarbon (9). The addition of water through a side-arm forces the lighter organic phase into a capillary tube where it is sampled with a syringe. With three extractions recoveries greater than 90% are obtained. Of course, a highly favourable solute partition coefficient and complete water immiscibility of the extracting solvent are vital to the success of this approach.
Single-Drop (10) and Suspended Microdroplet (11): These related techniques also rely on favourable partition coefficients. However, the single-drop approach may also be used in the headspace mode. Once optimized, these techniques can provide good analytical results.
Sorptive-Based Extractions: Since its introduction in the 1970s, SPE has become almost routine in many laboratories, especially in the pharmaceutical industry. In addition to the extraction selectivity offered by the stationary phase, substantial solvent savings may be made. Refinements and developments of other extraction technologies based on sorbents provide even greater green chemistry advantages. Among the most established sorptive methods that apply these advantages are the use of molecular recognition sorbents (12), SPME (13), stir-bar sorptive extraction (SBSE) (14), and supported liquid–liquid extraction (15).
Membrane Methods: Supported liquid membranes (16) and microdialysis also consume small volumes of extracting solvents for specialized applications.
Extraction Solvent Considerations
Regardless of extraction technique, one key green attribute is solvent use, as directly addressed in the fifth green chemistry principle. There are two approaches to addressing green chemistry concerns from the solvent perspective — minimization of solvent use and the use of "greener" solvents. With the exception of superheated water, each of the extraction techniques discussed in this supplement approaches this by reducing solvent use. However, solvent selection considerations are also important.
Solvent Selection: In addition to the physical-chemical properties of solvents, cumulative energy demand, toxicity, flash point, and similar characteristics should be addressed. For example, hexane is widely used as a nonpolar organic extraction solvent; yet it is the most neurotoxic of the n-alkanes (17) and hexane–acetone mixtures have even greater toxicological effects. Heptane is preferable as a nonpolar solvent. Many major pharmaceutical companies have led efforts to replace solvents. For example: Pfizer recommends the substitution of ethyl acetate, methyl-t-butyl ether, toluene, or 2-methyltetrahydrofuran for dichloromethane during extractions. These industrial efforts have led to the development of several methods to characterize "greenness" of solvents (18,19,20) and solvent guides. These reports also include summaries of computer-aided solvent selection and property tools.
Green Solvents: Several natural product-derived organic solvents are being explored for their green attributes. Jessop has called for the continued investigation into new solvents (21). Among emerging green solvents of interest are d-limonene (22), ethyl lactate (23), γ-valerolactone (24), 2-methyltetrahydrofuran (25), and cyclopentyl methyl ether (26).
Ionic Liquids and Deep Eutectic Solvents: The use of ionic liquids to extract solvents was recently reviewed (27). Emphasis was placed on single-drop microextraction, SPME, dispersive liquid–liquid microextraction (DLLME), hollow fibre-supported liquid membrane extraction, and SPE. While ionic liquids may be tuned for the application, have negligible vapour pressure, and possess high thermal stability, their high viscosity and toxicity (especially of methylimidazolium ionic liquids) render their widespread use somewhat limited. It will be interesting to see what developments occur over the next decade enabling utility of these intriguing solvents.
On the other hand, deep eutectic solvents (DESs) — formed by the interaction of, for example, hydrogen bond donors with quaternary ammonium compounds — offer several unique advantages compared with their ionic liquid cousins. DESs can be an order of magnitude, or more, less viscous, inexpensive, and nontoxic. Natural DES, made from sugars, water, and small acids have been reported as extraction media (28,29,30,31). Their high solubilization power for both polar and nonpolar compounds was demonstrated in the isolation of coumaroyls and other safflower compounds, non-water soluble bioactive natural products, gluten, starch, DNA, and quercetin. The properties of DES comprised of choline, chloride, or acetylcholine chloride with urea or glycerol have also been intensively studied (32). Solvatochromic studies indicated that these DES fall into the category of polar hydrogen bond-donating solvents because of their high ET (30) or ETN values. In addition, the effect of temperature and water content on polarity and Kamlet–Taft parameters of DESs was studied.
Summary
Modern sample preparation technologies grew up alongside, but separate from, green chemistry. However, the environmental aspects featured in these technologies parallel the 12 principles of green chemistry. Recent developments have tended to focus on reducing solvent usage in chemical extractions. Over the next decade or so, advances based on research into green solvents, ionic liquids, and deep eutectic solvents, as well as solvent cumulative energy demand and chemical toxicity will drive the green chemistry attributes of current and future sample preparation technologies.
Victor Essel works as a Postdoctoral Research Fellow in the Department of Chemistry and Biochemistry at South Dakota State University. His field of interest is in analytical chemistry and green chemistry, with specialty in the pretreatment of biomass for biofuel production, chromatographic separations, and analytical method development and validation.
Douglas Raynie is an Associate Research Professor at South Dakota State University. His research interests include green chemistry, alternative solvents, sample preparation, high resolution chromatography, and bioprocessing in supercritical fluids. He earned his Ph.D. in 1990 at Brigham Young University under the direction of Milton L. Lee. Direct correspondence to: Douglas.Raynie@SDSTATE.EDU
References
(1) P.T. Anastas and J.C. Warner, Green Chemistry: Theory and Practice (Oxford University Press, New York, USA, 1998).
(2) R.E. Majors and D.E. Raynie, LCGC North America 29(2), 118–135 (2011).
(3) R.E. Majors, LCGC North America 30(12), 1024–1031 (2012).
(4) Z. Takats, J.M. Wiseman, B. Gologan, and R.G. Cooks, Science 306(5695), 471–473 (2004).
(5) H. Chen, N.N. Talaty, Z.Takats, and R.G. Cooks, Anal. Chem. 77(21), 6915–6827 (2005).
(6) R.B. Cody, J.A. Laramee, and H.D. Durst, Anal. Chem. 77(8), 2297–2302 (2005).
(7) D.E. Raynie, G. Degam, B.A. Anderson, and K. Odegaard, "Greener Chelating Agents for Analytical Titrations," presented at the 14th Green Chemistry and Engineering Conference, Washington, DC., USA, (2010).
(8) C. Bendicho, I. De La Calle, F. Pena, M. Costas, N. Cabaleiro, and I. Lavialla, Trends Anal. Chem. 31, 50–60 (2012).
(9) D.A.J. Murray, J. Chromatogr. A. 177(1), 135–140 (1979).
(10) F. F. Cantwell and M. Losier, Comp. Anal. Chem. 37,297–340 (2002).
(11) L. Yangcheng, L. Quan, L. Guangsheng, and D. Youyuan, Anal. Chim. Acta 566(2), 259–264 (2006).
(12) H. Kataoka, Trends Anal. Chem. 22(4), 232–244 (2003).
(13) C.L. Arthur, L.M. Killam, S. Motlagh, M. Lim, D.W. Potter, and J. Pawliszyn, Environ. Sci. Technol. 26(5), 979–983 (1992).
(14) E. Baltussen, P. Sandra, F. David, and C.A. Cramers, J. Microcol. Sep. 11(10), 737–747 (1999).
(15) R. E. Majors, LCGC 24(2), 118 (2006).
(16) J.A. Jonsson, Comp. Anal. Chem. 37, 503–530 (2002).
(17) Agency for Toxic Substances and Disease Registry (July 1999). "Toxicological Profile for n-Hexane". Atlanta, GA: U.S. Department Of Health And Human Services.
(18) C.S. Slater and M. Savelski, J. Environ. Sci. Health A 42, 1595–1605 (2007).
(19) R. Gani, C. Jimenez-Gonzalez, and D.J.C. Constable, Comput. Chem. Eng. 29, 1661–1676 (2005).
(20) C. Jimenez-Gonzalez, A.D. Curzons, D.J. Constable, and V.L. Cunningham, J. Clean Technol. Environ. Policy 7, 42–50 (2005).
(21) P.G. Jessop, D.A. Jessop, D.Fu, and L. Phan, Green Chem. 14(5), 1245–1259 (2012).
(22) K. Faure, E. Bouju, P. Suchet, and A. Berthod, Anal. Chem. 85(9), 4644–4650 (2013).
(23) S. Aparicio and R. Alcalde, Green Chem. 11(1), 65–78 (2009).
(24) I.T. Horvath, H. Mehdi, V. Fabos, L. Boda, and L.T. Mika, Green Chem. 10(2), 238–242 (2008).
(25) D.F. Aycock, Org. Process Res. Dev. 11(1), 156–159 (2007).
(26) K. Watanabe, N. Yamagiwa, and Y. Torisawa, Org. Prcess Res. Dev. 11(2), 251–258 (2007).
(27) T.D. Ho, H. Yu, W.T.S. Cole, and J.L. Anderson, LCGC 31(2), 92–107 (2013).
(28) Y. Dai, G.J. Witkamp, R. Verpoorte, and Y.H. Choi, Anal. Chem. 85(13), 6272–6278 (2013).
(29) Y. Dai, J. van Spronsen, G. Witkamp, R. Verpoote, and Y. Choi, Anal. Chim. Acta 766,61–68 (2013).
(30) M. Francisco, A. van den Bruinhorst, and M. C. Kroon, Green Chem. 14(8), 2153–2157 (2012).
(31) Y.H. Choi, J. van Spronsen, Y. Dai, M. Verberne, F. Hollmann, I.W.C.E. Arends, G.-J. Witkamp, and R. Verpoorte. Plant Physiol. 156(4), 1701–1705 (2011).
(32) G. Degam and D.E. Raynie, Chimica Oggi (submitted).

Analysis of PFAS in Milk by LC-MS/MS
May 15th 2025Dairy milk is one commodity that can be impacted by environmental contaminants, such as PFAS, so it is important to implement extensive, robust, and accurate testing. In this work, a sensitive and reliable method was developed for the analysis of PFAS in milk by LC-MS/MS at levels as low as 0.01 µg/kg.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










