Sample Preparation for Chromatography: How Much Can Be Automated?
Special Issues
The author reviews the automation capabilities available, and some practical considerations to take into account when choosing to automate some or all of the sample preparation and handling steps that must be done before analysis.
Modern autosamplers and workstations possess a range of capabilities, in addition to simple liquid handling, that allow automation of sample preparation steps traditionally performed manually. Scientists in many laboratories are considering automating labour-intensive sample processing steps — such as weighing, filtration, dilution, solid-phase extraction, evaporation, and reconstitution. Most quantitative methods require preparation of calibration standards that can be as time-consuming as preparing the samples. This article reviews the automation capabilities available and some practical considerations to take into account when choosing to automate some or all of the sample preparation and handling steps that must be done before analysis.
Those of us who experienced the early days of high performance liquid chromatography (HPLC) may remember manual injections and strip-chart recorders on chromatographic instruments. In the mid-1980s rapid development of autosamplers, integrators, and personal computers (data systems) greatly simplified sample preparation and contributed to the development of modern automated chromatographic instrumentation.
Fifteen years later, in 1995, an article published in LCGC North America (1) provided insights from experts regarding sample preparation for chemical analysis, and predictions regarding where developments would head over the next decade. Increasing sample loads, the need for higher productivity in the face of decreasing analyst skill levels, and increasing issues with worker safety, regulatory constraints, and information management were all listed. Sound familiar?
Noteworthy were consensus opinions on the need for rugged and reliable automation systems that could be automated. It was agreed that future trends would include sample miniaturization and reduced solvent usage. Also, experts agreed that pressure from industry programmes and government agencies could play a significant role in stimulating development. Another major theme was the need for better, more universal communication between instruments to allow automation devices to communicate with any vendor's chromatography platforms.
Opinions differed regarding the importance of integrated (serial) sample processing versus batch processing, and the need for flexible (user-defined) robotic systems that provide a set of "tools" that could be customized versus specific systems designed to provide automated defined solutions. It was stated that developments in detection technology, particularly in the area of liquid chromatography–mass spectrometry (LC–MS), might reduce the need for extensive sample preparation.
A later article (1998) (2) on sample preparation for LC–MS–MS emphasized automation of three common procedures — dilute-and-shoot, solid-phase extraction (SPE), and liquid–liquid extraction — as well as post-injection techniques — column switching and on-line SPE— to manage the sample matrix. The authors also recognized the emerging need for "massively parallel" sample preparation and analysis driven by modern drug discovery practices such as combinatorial chemistry.
This review addresses the following questions: What driving forces are stimulating the continued development of the automation of sample preparation for gas chromatography (GC) and HPLC today? Where do we stand regarding current automation capabilities? What are the next developments on the horizon?
Defining Sample Preparation
One of the most formidable challenges to standardization and automation in the analytical laboratory is the broad range of sample types. Samples can be gaseous, liquid, solid, or a mix of phases (for example, biological tissues). Some sample types lend themselves to relatively straightforward collection of homogeneous samples — for example water, some liquids, and air samples. Other solid sample types, such as whole fruits and vegetables or production batches of pharmaceuticals, require physical homogenation of larger representative samples before reducing the sample size to a more easily manipulated volume. This article will address only the automation of processing steps for small, homogeneous, or homogenized samples.
Samples are often intact prior to sample preparation, but there are a few examples of sample collection devices that perform partial sample preparation as part of the process of collecting the sample. Air sampling onto adsorbent tubes concentrates analytes and simplifies transport. Similarly, solid-phase microextraction (SPME) (3) and stir-bar sorptive extraction (SBSE) (4) concentrate analytes onto extraction phases on fibres or stir bars, respectively, that can stabilize the analytes and facilitate transport. Although typically performed manually, these methods simplify downstream sampling and analysis, and can be automated with relative ease.
Automating Sample Introduction
Virtually all manufacturers of chromatographic instrumentation provide some type of automation for sample introduction into their systems and users expect this capability as a standard offering. Numerous companies also manufacture devices for sample introduction that can be interfaced with a variety of chromatographic platforms. Basic engineering decisions made about the core design of these systems often defines whether they are dedicated or flexible; compact or expandable; push-button-intuitive; or user-programmable. It is not possible to review all available instrumentation for sample preparation here. Instead, features of representative commercially available instruments are described. Given that upgrades and new designs are constantly released, this review will provide a snapshot in time of this continuously evolving field.
Modern autosamplers used for sample injection already include some sample preparation. Liquid injections, used almost universally for injections into LC and GC instruments, may not normally be considered sample preparation. However, when coupled with a programmed temperature vaporizing (PTV) inlet in a GC system, an autosampler can perform large volume injection (LVI) eliminating manual sample concentration or evaporation (5).
Likewise, autosamplers for headspace injection techniques — whether dedicated units (the Agilent 7694E or the PerkinElmer TurboMatrix) or flexible syringe-based instruments such as those based on the CTC PAL platform (CTC Analytics) — already transport, thermostat, and reproducibly transfer samples from the vial headspace into the GC inlet eliminating manual handling steps and interference from nonvolatile sample matrices. More specialized samplers and accessories from suppliers such as Dani, Tekmar, EST Analytical, OI Analytical, Gerstel, and others can automate dynamic headspace sampling techniques.
SPME is another automatable GC injection technique that includes sample preparation. A number of SPME innovations have been automated including:
- Pre- or post-extraction derivatization: The autosampler inserts the SPME fibre into a vial containing reagent either before or after exposure to the sample.
- Hot injection and trapping (HIT): The SPME fibre is inserted into a thermal desorption unit mounted above a cold inlet that allows refocusing of the analytes improving peak shapes for early eluting compounds.
New multi-fibre exchange modules for the CTC-type sampler use Supelco's new Fast Fit Fibre Assemblies (developed jointly with Chromline srl Prato) designed for easier field sampling and offer barcode labelling and stable transport after sampling. Modules are available that offer three preconditioned SPME fibres, or alternatively a fibre can be picked from a tray (preloaded in the field) and automatically desorbed into the GC inlet.
Thermal introduction techniques for GC (thermal desorption and thermal extraction) also provide some automated sample preparation capabilities. Thermal desorption is the process of heating a sampling device containing a sorbent medium, such as an air sampling adsorbent tube or phase-coated stir bar, on which analytes have been trapped and concentrated. Thermal extraction involves directly heating a solid sample in a tube under a gas stream to extract and concentrate the volatiles on an integral secondary trap (available from Markes International, Frontier, Perkin Elmer, or CDS Analytical) or in a cryogenically cooled inlet (Gerstel) from which they are transferred directly to the GC column. Direct thermal extraction requires small sample sizes (5–100 mg) of ideally finely divided powder or thin films, but can eliminate sample preparation steps such as liquid extraction and filtration. Accessories are available (from Gerstel and CDS Analytical) to automate gas switching during thermal extraction or pyrolysis (to allow heating in the presence of air for oxidation studies or addition of reagents to perform thermochemolysis (Gerstel, Frontier), for example to study lignin structure (6).
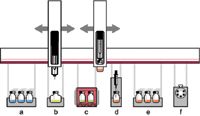
Dedicated accessory instruments are available for automating some types of sample cleanup and extraction as part of HPLC injection. Figure 1 shows a diagram of an autosampler platform with accessories for automated SPE using a disposable cartridge format. On-line SPE is available in a variety of formats and is used for rapid preconcentration and cleanup of samples. Valco Instruments lists a number of valving configurations, with formats ranging from regenerated and reused SPE cartridges configured as part of the sample loop to multivalve column switching systems such as those offered by Cohesive Technologies (Thermo Fisher Scientific). Alternatively, systems are available from Spark Holland and Gerstel that automatically exchange disposable SPE cartridges placed in-line between the injection valve and the analytical column.
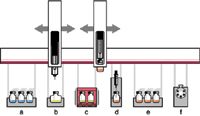
Figure 1: xyz-robotic autosampler configured for automated solid-phase extraction (SPE) before high performance liquid chromatography (HPLC) injection: a) sample vial storage, b) standards, c) incubation and agitation, d) SPE, e) eluate storage, and f) liquid chromatography (LC) instrument valve.
The need for rapid and widespread screening of blood samples has stimulated the development of methods for collecting dried blood spots (DBS) on filter paper cards. This technology was reviewed recently (7) and offers less invasive, cost-effective sample collection, and small sampling volumes. Also, when dry, samples are more stable and pose less risk to analysts handling the samples compared to liquid samples. Currently, the technique is mostly manual or semi-automated by punching out blood spots from the DBS cards. However, new instrumentation that extracts the spots on the card for further workup and analysis has been introduced. It will be of interest to see if major regulatory bodies will endorse this new form of blood sample handing.
Expanding Autosampler Capabilities
Samplers configured for headspace or SPME injection are typically preprogrammed with standard injection cycle options with few additional automation options. However, the capabilites of modern autosamplers for liquid injection can often be expanded. Samplers designed for GC injection typically use a single small (5–10-μL) syringe limiting the volume range that can be manipulated for other reagents, but they may still be able to automatically add internal standards to sample vials. Samplers injecting into HPLC valves using the loop overfill method, or GC autosamplers performing LVI, may be able to use larger syringes to add relevant volumes of internal standards, derivatizing reagents, or sample modifiers (salts, acids, or bases) as part of the automated sample processing steps.
Versatile Prepstations
In the past 10 years robotic xyz samplers capable of GC or HPLC injection have become the industry standard, although lower-cost dedicated autosamplers are still quite common. The evolution of robotic samplers has been driven by the addition of specialized hardware modules, and the development of versatile, user-friendly software. One of the first steps in this development was to mount two robotic samplers one above the other (referred to as a "twin" or "dual-rail" configuration). CTC Analytics then more recently created a longer autosampler platform (PAL-xt) to accommodate more accessories and, soon after, integrated two injection units into a single rail xyz sampler. Two different syringe types can be mounted simultaneously, allowing a wider range of sample processing options. This configuration has been used to automate a variety of headspace GC methods with liquid internal standard or reagent addition including blood alcohol (8), and cyanide in drinking water (9).
Alternatively, specialized liquid delivery modules are available that can deliver millilitre volumes of a dilution solvent. A configuration using a dilutor module from CTC Analytics was used to automate ASTM standard method D6584-07 for biodiesel (10). The widely practiced dilute-and-shoot technique used in LC–MS–MS to reduce interference and ion suppression can also be automated by using autosamplers capable of handling both larger diluent and smaller microlitre volumes.
Automated manipulation of the sample is not the only benefit provided by these flexible prepstations. The additional liquid-handling capability has been routinely used for serial dilution and automated preparation of calibration standards for liquid, headspace, and SPME injection methods (11,12). This is particularly beneficial to automating matrix-matched standard preparation — blank sample matrix samples are processed at the same time as test samples, before being spiked with standards. For example, automated extraction and analysis of pesticides using the QuEChERS (quick, easy, cheap, effective, rugged, safe) approach using matrix-matched standards improved quantification (13).
The proliferation of flexible xyz robotic autosamplers created the need for software control that was user-programmable so that customized processing steps could be created by the analyst. Firmware and software provides the initial framework and tools for customization of autosampler actions. For example, CycleComposer software (CTC Analytics) provides detailed control features enabling the analyst to create customized actions, but requires an understanding of the underlying macro language. Instrument manufacturers subsequently provided drivers or other integrated control of these samplers within their proprietary chromatographic platforms, but most of the embedded control is for standard injection modes, with relatively little flexibility.
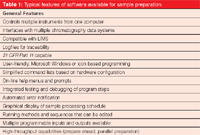
Table 1: Typical features of software available for sample preparation.
There is clearly a need for user-friendly, flexible software control of customizable autosampler platforms that can communicate effectively with a variety of different chromatographic platforms such as ChemStation (Agilent Technologies), MassHunter (Agilent Technologies), Analyst (AB Sciex), Xcalibur (Thermo Fisher Scientific), Millenium (Waters Corp.), and ChromaTOF (Leco). Third party instrument distributors focused on front-end sample automation may be able to meet this need. Typical software features currently available on some of these instruments are listed in Table 1. For example, Maestro software (Gerstel) provides a user-friendly Microsoft Windows-based programming interface to control the CTC autosampler platform. Standard injection modes are programmed with on-screen help and prompts for recommended parameter ranges. A list of typical sample preparation modules from a variety of vendors is provided in Table 2.

Table 2: Sample preparation hardware modules available for several platforms.
Steps To Automate
Highlighted here are a few simple steps that can be automated and that are likely to become much more commonly integrated into routine automated operations. Coupling sample preparation with GC analysis was recently reviewed in depth (14).
Liquid–liquid extraction is often used before injection, and when large sample volumes are not needed, in-vial liquid–liquid extraction may become more common. Mixing devices are available to achieve efficient extraction that can achieve orbital mixing speeds of 750 to 3000 RPM. When smaller (2-, 4-, or 10-mL) vials are used the extracting organic solvent may be more or less dense than the aqueous phase, and the layer to be injected onto the instrument can be selected by adjusting the penetration depth of the autosampler syringe needle. When larger (20-, 40-, or 100-mL) vessels and dense solvents are required, the sampler must allow liquid to be withdrawn from the lower layer. One alternative approach is membrane-assisted solvent extraction (MASE) that uses a small polypropylene bag filled with the extraction solvent suspended in the vial (15). Dense solvents can be used because the bag holds the liquid in a position easily accessible by the autosampler syringe.
Evaporation and solvent exchange are common procedures to dissolve analytes into a solution compatible with the HPLC eluent. Single- and multi-position evaporation modules are available on some instruments allowing full automation through serial- or semi-batch processing.
Filtration is often the final step when manually processing samples for injection, particularly before HPLC or ultrahigh-pressure liquid chromatography (UHPLC), to prevent blocking the narrow orifices in valves and connection tubing. Particulates may already be present in samples or may form after experimental steps — in this instance, pH adjustment, solvent addition, various digestion procedures, or even the simple dilute-and-shoot approach. In-vial devices are available (Whatman, Thomson) that allow manual filtration of the sample as it is placed into the vial. An accessory is available from Leap Technologies that allows the CTC autosampler to perform the filtration with these devices. Filtration using conventional syringe filters can also be automated, although care must be taken to ensure particulates do not contaminate the autosampler syringe or needle.
The newest autosampler platform from CTC Analytics is known as the PAL RTC, or Robotic Tool Change system. In addition to automating standard sample introduction capabilities — liquid, headspace, and SPME — the RTC can automatically exchange tools (in this instance, syringe types) enabling a single injection unit system to carry out multiple functions without being limited by one single syringe type. As new autosampler platforms enter the market new software control will be needed, but this will take time.
A number of sample preparation steps required immediately before sample injection can now be automated, allowing "just in time" sample preparation. For this to be efficient, sample preparation steps must be completed during the chromatographic separation of the previous sample, to prevent automated sample preparation from becoming a bottleneck and limit throughput.
Some software (Gerstel Maestro) is capable of automatically overlapping or grouping sample preparation steps to maximize throughput without the need for cumbersome analyst programming. For example, automated saponification and esterification for analysis of fatty acid methyl esters (FAMES) and pain management drug screening in urine have been automated (16,17).
Standalone Workstations
A number of high-throughput analysis methods are being developed based on fast GC, HPLC, or UHPLC where the analytical runtime is too short to allow efficient coupling of sample preparation steps. For example, high throughput LC–MS–MS analyses may be completed in a couple of minutes. Automated sample preparation supporting these types of analyses have therefore been taken off-line.
Off-line automation allows interfacing with equipment or processing steps that are currently incompatible with the chromatographic system. Application examples include high-speed centrifugation, or weight verification after liquid transfer using a balance. Weighing solids and powders typically requires more sophisticated robotic modules. Sometimes the sample load for one analysis type may not justify the expenditure for automation, but a single workstation platform automating several sample preparation procedures, potentially "feeding" several different analytical instruments, may fit into a laboratory workflow.
The Agilent 7696A Workbench is an example of a standalone sample preparation platform designed to provide flexible liquid handling and some additional features including heating, cooling, mixing, and weighing. The system is based on handling small volumes in 2-mL vials reducing solvent use and waste generation; but may require modification and revalidation of the analytical method to accommodate the reduced sample size. The Agilent Workbench has been used to miniaturize and automate liquid–liquid extractions of drugs in plasma, perform serial dilutions to prepare standards for calibration, and fatty acid methyl ester (FAME) analysis in biodiesel (18,19).
Gilson offers a number of systems for off-line liquid handling and SPE. The Gilson GX-281, for example, is based on a syringeless pump design and can function as a standalone workstation or can be coupled to an HPLC instrument by incorporating a proprietary injection valve in the configuration. It is designed to handle samples in a variety of test tubes or scintillation vials, offering automated valve control to access up to five solvent reservoirs and six rinse solvents, and all flowpaths are constructed of biocompatible materials. With additional accessories, tubes can be moved to a compatible balance option. Other models such as the GX-274 allow automated positive pressure elution SPE in 1-, 3-, and 6-mL cartridge formats.
Finally, there is a class of dedicated robotic systems designed for either complete automation of a specific procedure or for high-throughput, parallel processing of small sample volumes, often in a 96- or 384-well plate format. Tecan, Tomtec, and Zinsser NA provide a range of robotic systems for liquid handling. Dedicated, robotic workstations are available from PerkinElmer (Janus workstation), Agilent, and others. Teledyne Tekmar has developed the AutoMate-Q40 for complete automation of the QuEChERS extraction for pesticides from fruit, vegetables, and other foodstuffs. With this system, homogenized sample is prepared and appropriate amounts of sample (typically 15 g) in 50-mL conical centrifuge tubes are loaded onto the AutoMate-Q-40. The automated system adds solvents, internal standards, and solid salts, performing all mixing and centrifugation steps. A portion of the upper acetonitrile layer is removed automatically and added to a tube containing dispersive SPE sorbent that is mixed and centrifuged. A portion of the cleaned-up extract is then transferred to a final sample tube from which it can be placed into a sample vial for GC–MS analysis.
Conclusion
The key themes cited by the experts almost two decades ago have largely been realized in modern instrumental sample preparation methods. We now use smaller samples and less organic solvent and handle a wider variety of tasks automatically with instrumentation providing better traceability and communicating more universally with different vendor's platforms. In the areas where the experts envisioned parallel paths, we have seen parallel development, with the growth of both serial "just in time" processing and batch type "workstation" automation systems, as well as the development of both dedicated analyzers and flexible, customizable platforms to meet quickly evolving needs.
One clear challenge is to develop flexible software tools that can keep up with changing computer operating systems and chromatography platforms. There is a need for software to take advantage of the hardware capabilities available, combining them in unique ways to address emerging issues in the analytical laboratory.
Pressure to increase capacity to screen more samples faster and more accurately continues from a number of areas (such as pharmaceutical, food and beverage, medical, and biological). High-throughput and fast analyses are likely to require more development in standalone workstations that can prepare samples off-line. Automation is unlikely to replace all manual sample preparation in the laboratory, but is likely to become an increasingly common part of modern methods.
Edward Pfannkoch is the Director of Technology Development, North America for Gerstel Inc. He has over 25 years experience in chromatography method development, automation, and sample preparation. He has authored three book chapters, numerous scientific publications, and dozens of corporate technical reports regarding sample preparation, automation, and analysis. Direct correspondence to: EAPfannkoch@gerstelus.com
References
(1) Ronald E. Majors, LCGC 13(9), 742–749 (1995).
(2) J. Henion, E. Brewer, and G. Rule, Anal. Chem. 70(19), 650A–656A (1998).
(3) R. Belardi and J. Pawliszyn, J. Water Pollut. Res. J. Can. 24, 179–191 (1989).
(4) E. Baltussen, P. Sandra, F. David, and C.A. Cramers, J. Microcol. Sep. 11, 737–747 (1999).
(5) J. Staniewski and J.A. Rijks, J. Chromatogr. 623, 105–113 (1992).
(6) T.R. Filley, R.D. Minard, and P.G. Hatcher, Organic Geochemistry 30(7), 607-621 (1999).
(7) J. Déglon, A. Thomas, P. Mangin, and C. Staub, Anal. Bioanal. Chem. 402(8), 2485–98 (2012).
(8) C.L. Morris-Kukowski, E. Jagerdeo, J.E. Schaff, and M.A. LeBeau, Journal of Chromatography B 850, 230–235 (2007).
(9) Method ME355.01, Revision 1.0 "Determination of Cyanide in Drinking Water by GC/MS Head-space Analysis" May 26, 2009. [Available at https://www.nemi.gov or from James Eaton, H & E Testing Laboratory, 221 State Street, Augusta, ME, USA, 04333].
(10) J. Stuff and J. Whitecavage, Gerstel Application Note 1/2010 (2010).
(11) K. Summerhill and J. Angove, Anatune Chromatography Technical Note No AS51 [Available from: http://www.anatune.co.uk/resources/as51].
(12) J. Angove and K. Summerhill Anatune Chromatography Technical Note No AS45 [Available from: http://www.anatune.co.uk/resources/as45].
(13) V. Settle, F. Foster, P. Roberts, P. Stone, J. Stevens, J. Wong, and K. Zhang, Gerstel Application Note 4/2010 (2010) [Available from: http://www.gerstelus.com/applications_category.php?id=65].
(14) H. Lord and E. Pfannkoch, Comprehensive Sampling and Sample Preparation, J. Pawliszyn Ed. (Elsevier Academic Press, 2012).
(15) B. Hauser, P. Popp, and E. Kleine-Benne, J. of Chromatogr A963, 27–36 (2002).
(16) J. Stuff and J. Whitecavage, Gerstel Application Note 3/2013 (2013) [Available from: http://www.gerstelus.com/applications_category.php?id=93].
(17) O. Cabrices, F. Foster, J. Stuff, E. Pfannkoch, and W. Brewer, Gerstel Application Note 1/2012 (2012) [Available from: http://www.gerstelus.com/applications_category.php?id=88].
(18) A. Macherone and J. McCurry, "Automating Sample Preparation for the GC Analysis of Biodiesel using Method EN14105:2011," presented at the ASMS Conference on Mass Spectrometry and Allied Topics, Minneapolis, Minnesota, USA, 2013.
(19) S. Salman, S. Palaniswamy, and S. Babu "Extraction of Drugs in Plasma by Automated Liquid-Liquid Extraction For Downstream Analysis," presented at the ASMS Conference on Mass Sprectrometry and Allied Topics, Minneapolis, Minnesota, USA, 2013.

Determining the Effects of ‘Quantitative Marinating’ on Crayfish Meat with HS-GC-IMS
April 30th 2025A novel method called quantitative marinating (QM) was developed to reduce industrial waste during the processing of crayfish meat, with the taste, flavor, and aroma of crayfish meat processed by various techniques investigated. Headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) was used to determine volatile compounds of meat examined.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








