Free Excel Software for Performing Virtual Liquid Chromatography
The “Practical HPLC calculator” is a new free simulator using Microsoft Excel® for virtually learning the concepts of high performance liquid chromatography (HPLC) and ultrahigh-pressure liquid chromatography (UHPLC). It has been developed by researchers from the University of Geneva in Switzerland to teach LC concepts using a virtual model calculator. This software is useful for students, professional educators, and trainers to teach and perform virtual HPLC experiments and to accomplish a limited number of real experiments using an HPLC instrument.
Following a very informative recent article from Charles A. Lucy published in LCGC North America (1) about simulation software available for chromatography, we wanted to highlight the existence of a new simulator for virtual liquid chromatography (LC) developed by researchers from the University of Geneva in Switzerland. With the current COVID-19 pandemic resulting in limited access to the laboratory, the existence and availability of such a tool can be extremely beneficial for teaching activities.
“Practical HPLC calculator” is a free Excel tool that can be downloaded directly from this website: https://ispso.unige.ch/labs/fanal/practical_hplc_simulator:en. There are two versions of this Excel spreadsheet available. The first version does not contain any macro, which is designed for those who are not allowed to use macro in Excel, for safety reasons. The second one includes several macros that can reset all the values to default by clicking on one button, configure the calculator to mimic a standard high performance liquid chromatography (HPLC) instrument, and configure the calculator to mimic a standard ultrahigh-pressure liquid chromatography (UHPLC) system. The second version with macro should be preferentially used whenever possible.
The main purpose of this calculator is to teach LC in a virtual way. Therefore, this calculator could be at the center of “virtual practical works” for students that are in college or currently undertaking internships. It can also be used by professional trainers during education courses to highlight some particular concepts related to HPLC, using simulated chromatograms.
In our School of Pharmaceutical Sciences at the University of Geneva, we use this tool to perform hybrid practical works. Students spend part of their time doing HPLC experiments virtually on the calculator (without the need to perform repetitive experiments), and once they master the basics of HPLC, they can do a very limited number of real experiments on a HPLC instrument to see how it works in practice. This is a valuable solution to reduce the number of instruments necessary in the practical work laboratory and the cost of operation.
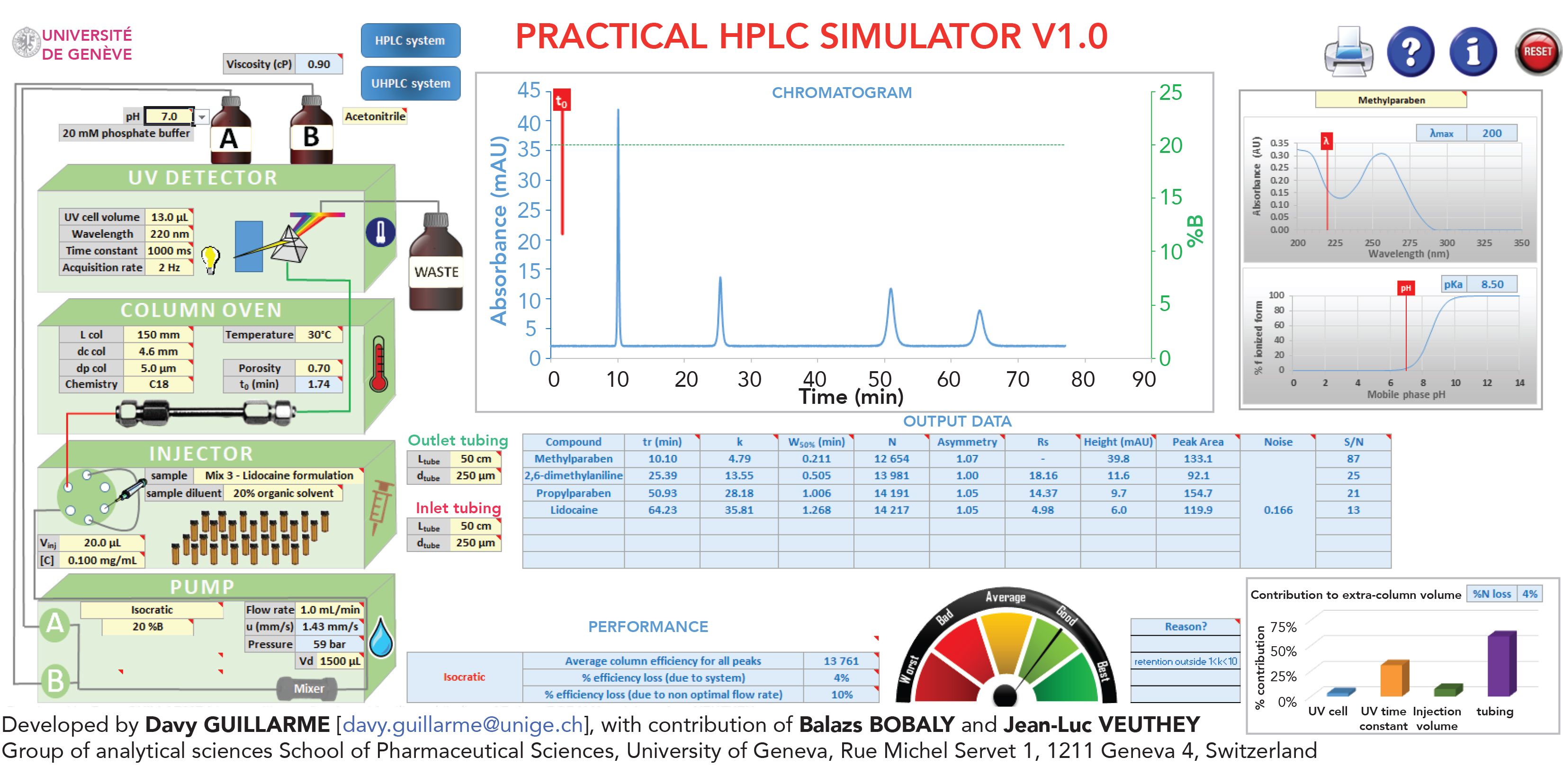
This software allows an easy understanding of the impact of all chromatographic parameters on the final separation. For this purpose, the user has a choice between seven different mixtures of representative compounds composed of a number of molecules comprised between four and seven. Among the available mixtures, we can find parabens, nonsteroidal anti-inflammatory drugs, lidocaine formulation, cannabinoids, lipo-soluble vitamins, salbutamol, and related impurities, along with doping agents.
The software can also simulate the chromatogram obtained in any condition. The user will be able to tune the following parameters: mobile phase (pH and type of organic modifier), pump (elution mode, composition, flow rate, dwell volume), injector (volume injected, concentration, sample type, sample diluent), column (length, diameter, particle size, chemistry, porosity), oven (temperature), ultraviolet (UV) detector (cell volume, wavelength, time constant, data acquisition rate), and tubing used in the system (length and diameter).
Then, the software will show the corresponding simulated chromatogram, and the user will also have access to a variety of additional parameters, including retention times, retention factors (k’), peak widths at half height, efficiencies (N), peak capacities, asymmetries, resolutions, peak heights, peak areas, and background noise for all compounds under the simulated conditions. The software will also evaluate the efficiency loss because of the instrument (extra-column effects), the overall quality of the separation and will provide the UV spectrum and ionization profile of the analyzed compounds.
It is important to mention that more than 3500 practical experiments were performed (which cumulatively totaled to 37 molecules, four columns, two organic modifiers, four mobile phase pH, two mobile phase temperatures, and two gradient times) to develop this simulation tool and mimic in the best possible way the chromatographic behavior of all the compounds that can be analyzed with this tool. All these experiments were performed using an ultraviolet diode array detector (UV-DAD) from 200 to 350 nm to have reliable sensitivity at various wavelengths, as well as realistic background noise and baseline drift. Based on the practical experiments, the retention models (log kw and S) were calculated to simulate any isocratic or gradient conditions. Last, but not least, van’t Hoff parameters were also obtained from the experiments performed at two temperatures, which allowed the simulation of chromatograms at any other temperature suggested by the user.
FIGURE 1: Screenshot showing features of the Excel tool “Practical HPLC calculator.”
For those involved in teaching, a document can be downloaded from the same address: https://ispso.unige.ch/ labs/fanal/practical_hplc_simulator:en. It shows an example of a virtual practical work in HPLC that we have developed and can be used by anyone interested. It requires approximately one full day for a student to do this virtual practical work including the writing of the corresponding report summarizing the findings.
To develop some new HPLC exercises with this software, below is a nonexhaustive list of topics that can be explored with this calculator depending on the level of the users.
For a beginner, they can explore the following topics: a) the impact of modification of percent organic modifier on overall performance; b) the impact of the organic modifier nature on retention and selectivity; c) the impact of column chemistry on resolution; d) playing with gradient conditions to tune separation; and e) the impact of analytical conditions on back pressure.
For intermediate users, they can explore the following topics: a) the quantitative performance evaluation for an analytical method; b) impact of mobile pH on the final separation and relationship with ionization profile of the compounds; c) comparing HPLC and UHPLC conditions; d) impact of UV time constant and wavelength on sensitivity; and e) impact of mobile phase flow rate on efficiency (van Deemter curves).
Finally, advanced users can explore the following topics: a) the impact of instrumentation (extra-column band broadening) on the final separation; b) the evaluation of peak capacity in gradient mode based on the chromatographic conditions; c) understanding the impact of sample diluent nature on peak broadening; and d) assessing the impact of dwell volume in gradient mode.
We hope you will find this Excel tool useful, and if you have any comments to improve it, drop an e-mail to davy.guillarme@unige.ch. Since it is freely available, please do not hesitate to spread this tool to make it even more popular.
References
(1) C.A. Lucy, LCGC N. Am. 38(8), 456–468 (2020).
Davy Guillarme, Balazs Bobaly, and Jean-luc Veuthey are with the Institute of Pharmaceutical Sciences of Western Switzerland (ISPSO) and the School of Pharmaceutical Sciences at the University of Geneva in Geneva, Switzerland. Direct correspondence to: davy.guillarme@unige.ch.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).









