Flame Ionization: GC's Workhorse Detector
LCGC North America
As the flame ionization detector (FID) approaches its 60th anniversary in 2017, this installment examines the crucial role that it has played and continues to play for all types of gas chromatography. Without the FID, the early development of gas chromatography (GC) would have proceeded more slowly especially in the petroleum industry and related hydrocarbon application areas.
As flame ionization detection (FID) approaches its 60th anniversary in 2017, this installment examines the crucial role that it has played and continues to play for all types of gas chromatography (GC). Without FID, the early development of GC would have proceeded more slowly, especially in the petroleum industry and related hydrocarbon application areas.
Flame ionization detection (FID) is without a doubt the most often used gas chromatography (GC) detection method. While the older thermal conductivity detection (TCD) responds universally, it lacks the sensitivity and linearity that FID can deliver. Indeed, it is fortuitous that FID responds so well to the chemical moieties that constitute a majority of compounds of interest across many disciplines. The development and widespread acceptance of FID strongly boosted the rapid growth of GC, especially with capillary columns, during the early years.
A Brief History of FID
The invention and development of FID is a story of simultaneous multiple discovery in two locations by researchers who were unaware of each other’s efforts to improve on an existing detector (1,2). The predecessor to the flame ionization detector was the hydrogen flame detector, described by R.P.W. Scott in 1955, that measured the temperature increase of a hydrogen flame as GC column eluant passed through. The hydrogen flame detector was not particularly more sensitive compared to the ubiquitous thermal conductivity detector, and in 1957 two separate research groups began to investigate how to improve upon it: the Central Research Laboratories of Imperial Chemical Industries of Australia and New Zealand (ICIANZ), and the Department of Physical Chemistry of the University of Pretoria in South Africa.
Starting in early 1957, R.A. Dewar and I.G. McWilliam of ICIANZ endeavored to measure the ion current in the hydrogen flame instead of its temperature, while at about the same time V. Pretorius at the University of Pretoria measured the changes in the resistance across the hydrogen flame as peaks were eluted. Both groups submitted papers to Nature in late 1957. Pretorius’s paper was published first, but only because of delays in the editorial process around Dewar and McWilliam’s submission. In the end, it was the ICIANZ group that continued to develop FID, characterizing and optimizing several configurations and presenting a detailed report at the Second International GC Symposium held in Amsterdam, the Netherlands, in May 1958.
This event created large interest in FID and a number of additional investigators began to work with it, in particular with the then newly developed capillary columns. The relatively low flow rates and small sample capacities of capillary columns, compared to the prevalent packed columns, had stymied their wider adoption. The higher sensitivity and tolerance for low column flow rates in FID made it an ideal match for capillaries. FID was soon made available in the Perkin-Elmer Model 154-C GC system introduced at the 10th Pittsburgh Conference in 1959. Arguably the first commercialization of FID, it was soon followed by offerings from other companies. The Wilkens Aerograph Model 600 “Hy-Fi” GC system, introduced in 1961, reportedly was named for its hydrogen flame ionization detector. Wilkens Instruments was the predecessor to Varian’s chromatography division.
ICI obtained patent rights for the flame ionization detector in Australia as well as in a number of other countries including the United States, and the company subsequently issued licenses to as many as 37 instrument companies. More than 60,000 detectors were manufactured under license from ICI. Subsequent chromatography-related FID patents were obtained by Shell, Hewlett-Packard, Perkin-Elmer, Beckman, Pye Ltd., Varian, Microsensor Technology, and Phillips Petroleum, to name a few. Innovations around FID continue today as well, with some of the most recent work encompassing supercritical fluid chromatography (SFC) and microcolumn GC.
Nearly 60 years after its invention, FID continues to be the dominant GC detection method. While benchtop and portable mass spectrometric detection provides more information via spectral analysis, and selective detection methods such as electron-capture detection (ECD) offer unparalleled sensitivity and selectivity, FID brings sensitivity, low cost, simplicity, and reliability to GC detection.
Nuts and Bolts
A fraction of the carbon-based compounds that pass through the FID flame are ionized, something like one in 10,000 (3). In most modern designs, the electrons formed inside the detector are impelled by an electric potential toward a collector electrode, producing a minute current on the order of picoamperes (10-12 A). This current is converted to a voltage, filtered, and amplified as required. Even though not all of the carbon molecules are ionized, FID can deliver a limit of detection on the order of a few picograms of carbon per second.
The internal arrangement of a typical flame ionization detector is shown in Figure 1.
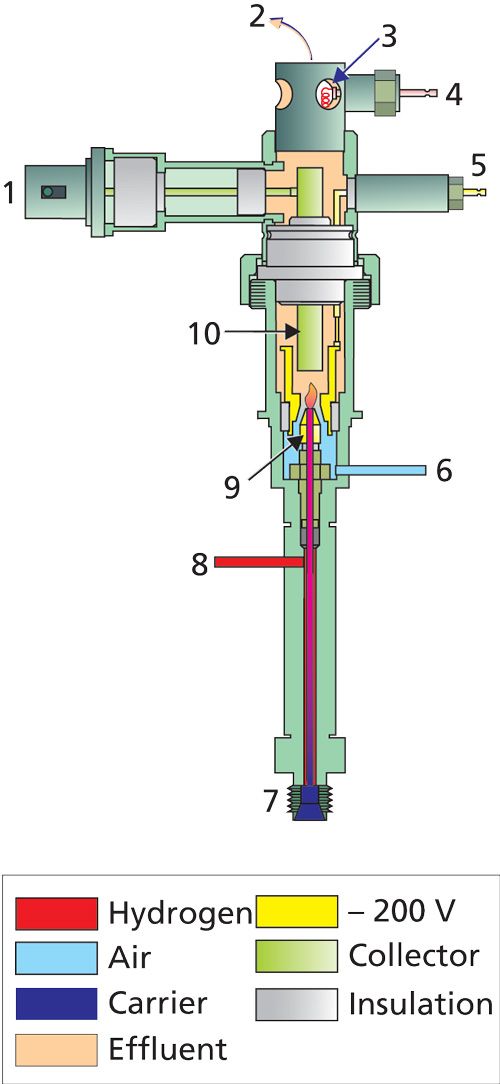
Carrier gas from the column enters at the bottom of the detector and is mixed with hydrogen combustion gas plus optional makeup gas in the area below the flame jet. This mixture is then combined with an excess of air and burned just above the jet tip. A negative polarizing potential is applied between the jet tip and a collector electrode; as electrons are formed they are accelerated across the jet tip–collector gap by the electric field. Depending on the detector design, either the collector or the jet tip is kept at ground potential; Figure 1 shows a grounded collector design with the electrometer input at virtual ground and the jet tip charged to -200 V. Air, carbon dioxide, and water exhaust gases are vented from the top of the detector body. In most modern FID systems a glow plug operates momentarily to ignite the flame.
The electric current from the collector is converted to a voltage by an electrometer and then is amplified, filtered, digitized, and processed as required. Signal zeroing, scaling, and attenuation are performed in the instrument firmware. The detector signal is filtered to remove unwanted high-frequency noise. Noise is produced by instabilities in the flux of ionizable impurities in the carrier gas, by the flame itself, by the electronic circuit, and by induction of stray electromagnetic signals from, for example, nearby cell phones. Excessive noise usually is the result of contamination from a buildup of carbon particles in the detector, poor electrical connections, or incorrect operational settings.
Sensitivity
FID sensitivity depends on the combustion gas flow rates, the carrier gas flow rate, the flame jet exit diameter, the electric field formed by the relative positions and shape of jet and collector, and to a lesser degree the detector temperature.
Combustion Gas Flow Rates
Combustion gas flow rates must be set correctly for proper detector operation; follow the manufacturer’s recommendations for air and hydrogen flow settings. In general, the air–hydrogen ratio should be approximately 10:1. A hydrogen flow rate of 30–45 mL/min with a corresponding air flow of 300–450 mL/min is common with standard laboratory detectors. FID sensitivity will be reduced if the hydrogen flow deviates above or below optimum. The linear dynamic range (LDR) is affected by hydrogen flow: higher flows tend to reduce the LDR. There is little reason to operate a flame ionization detector far away from the manufacturer’s gas flow settings; they have been carefully optimized for that specific detector. Air flow is less critical than the hydrogen flow, but too much air will destabilize the flame causing noise and possible flame-out. Too little air will reduce sensitivity and shorten the LDR.
Caution
Hydrogen is highly flammable and can cause a serious explosion if allowed to build up in an enclosed space such as the GC oven. Never turn on the hydrogen flow without a column or plug fitting attached to the detector base to prevent hydrogen from leaking into the oven. Available GC oven hydrogen monitors provide an additional safety level by shutting off hydrogen flow if dangerous levels are detected.
Carrier-Gas Flow Rate
The carrier-gas flow rate is an important consideration for detector sensitivity. FID does not require any special attention to flow considerations with standard 1/8–in. diameter packed columns or 0.53-mm and greater capillary columns operating at or above 8–10 mL/min. When capillary or micropacked columns are operated closer to their optimum flow rates of less than 5 mL/min, FID can benefit from the addition of makeup gas to the carrier stream before it enters the jet area.
Carrier-gas type, other than hydrogen, does not materially affect detector operation. Hydrogen, while seldom used for packed column carrier gas, is commonly used with capillary columns and has advantages that have been discussed in detail in “GC Connections” (4,5) and many other articles, conference talks, and seminars. Hydrogen carrier gas gives a wider range of optimum linear velocities, is less expensive than helium, and may be generated from water on demand with an appropriate hydrogen generator.
Since FID performance is affected, the hydrogen flow rate through the detector should be kept at an optimum level by considering the total flow. With a hydrogen carrier gas flow rate of 5 mL/min, for example, detector hydrogen flow should be reduced correspondingly by 5 mL/min. Carrier flow rates may change as the column oven temperature changes, depending on the mode of carrier control. Electronic pressure control systems can maintain constant total hydrogen flow through the detector by adjusting FID hydrogen dynamically as capillary column flows change during temperature or carrier-gas programming.
Makeup gas has two important effects. First, it establishes an optimum gas flow rate through the jet when carrier flow is low, which keeps the FID system operating at the best sensitivity and LDR. Second, for some detectors makeup flow sweeps out the area under the jet and inside the detector base, alleviating any peak broadening that might be produced as capillary peaks encounter larger-diameter internal passageways. With FID, use carrier gas as makeup gas. If hydrogen carrier gas is used, consider nitrogen makeup gas if maintaining the total hydrogen flow from three sources-carrier, makeup, and flame hydrogen-is impractical. Typical makeup gas flow rates are in the 10–20 mL/min range, but in all cases follow the manufacturer’s recommendations.
Detector Temperature
The sensitivity of a flame ionization detector does not depend strongly on its temperature, provided some conditions are met. The appropriate FID temperature is determined by the greater of a minimum temperature of 150 °C for stable detector operation, and a minimum temperature of approximately 20–50 °C above the highest column temperature, up to the maximum permitted detector temperature. FID produces a large amount of water vapor that may condense in the cooler upper areas around the collector if the detector base temperature is less than about 150 °C: this condensed water vapor can produce noise and baseline drift. On the other hand, the detector base must be hot enough to prevent condensation of peaks as they are eluted from the column, so it should be kept somewhat warmer than the highest operating column temperature. Some GC systems have an insulating cap that surrounds the detector column fitting. To avoid cold spots, always make sure to reinstall the cap after connecting a column to the detector.
If a capillary column is installed with its end inserted into the detector base up to the jet, and is operated at oven temperatures approaching the column’s maximum rated temperature, it is possible that the end of the column will be overheated if the detector base is significantly warmer. Such overheating can produce excessive detector noise from decomposing stationary phases, cause solute adsorption onto subsequently exposed column surfaces, and reduce column lifetime. A capillary column detector adapter that positions the end of the separation column in the oven and conducts carrier gas flow along a deactivated connector into the detector jet will help alleviate such problems.
Care and Feeding
Carrier and combustion gas purities and flow rates as well as detector and column temperatures are all important considerations when setting up a flame ionization detector. Be sure that all gases are of sufficiently high purity. Then, with the instrument turned on but unheated, set the required gas flows. Finally, heat the injector, detector, and column to their operating temperatures and ignite the flame.
Gas Source
Flame ionization detectors are sensitive to hydrocarbon impurities that may be present in gas cylinders or connecting tubing. Impurities in the combustion gases will cause increased detector noise levels as well as higher baseline signal levels. Hydrocarbon filters are highly recommended for installation at the external GC bulkhead fittings for air, hydrogen, makeup gas, and, of course, the carrier gas. It’s not necessary to remove oxygen from the FID hydrogen stream, but an oxygen filter on the carrier line is always recommended, certainly if hydrogen is used for carrier gas as well.
Hydrogen for FID alone should be of 99.995% purity or better. If used for carrier gas then 99.999% or better purity is best. There are several excellent commercial hydrogen generators that can produce sufficient carrier-grade hydrogen to supply dual flame ionization detectors plus one or two carrier channels with split injectors. If an electrolytic hydrogen generator is used be sure the water you add is free of hydrocarbon impurities.
Air for FID should contain less than 100 ppb of total hydrocarbon impurities. Besides standard compressed gas tanks, various suitable purified air generators are available with capacities ranging from a couple of chromatographs up to an entire laboratory’s worth. Older air compressors or so-called “house” air supplies should not be used with GC systems except to supply operating pressure for pneumatic valve actuators.
Carrier-gas purity is also important for proper FID operation-with or without makeup gas. Makeup gas impurities affect FID in much the same manner as combustion-gas impurities. Even without makeup gas, impurities in the carrier gas can eventually pass through the column and onto the detector. In temperature-programmed operation, such impurities may appear as broad ghost peaks during a run or as a steadily rising baseline similar to column stationary-phase bleed. In isothermal operation impurities may appear as a slowly rising baseline with increasing noise, often over a period of hours to days.
Connecting tubing from the gas source to the instrument also can sometimes cause problems with contamination. Be sure to use copper or stainless steel tubing specially cleaned for chromatographic applications. Never use plastic tubing because significant amounts of plasticizer or monomer may be present. In addition, plastic tubing is permeable to atmospheric oxygen. Avoid leakage by ensuring that all fittings and ferrules are in good shape and not over-tightened.
Setting FID Flow Rates
Two situations arise when setting FID flow rates depending on whether the gases are electronic pressure controlled (EPC) or manually controlled. With EPC systems the flows are set on the instrument keypad. Don’t assume, however, that the flows are correct-regular flow validation is highly recommended. I like to measure the detector flow rates anyway. Be careful to enter the related settings that control the carrier-gas mode of operation, makeup gas flow, and the identity of the gases in use. Also, bear in mind that in some GC systems with manual detector gas controls, flow rates depend on the supply gas pressure-if the pressure changes then the detector flows should be recalibrated.
For manually controlled detector gases, as well as when directly measuring detector gas flow rates, it’s easiest to operate with the column connection in the oven blocked off with a blank ferrule or plug fitting. If the column is installed then carrier flow should be enabled for capillary column installations where the column end is in the detector; in this situation the operator will need to correct measured combustion gas and makeup flows for the column flow rate.
To measure detector flows, attach a calibrated flowmeter to the detector exit with the appropriate adapter and turn off the air, hydrogen, makeup, and carrier-gas flows. Hydrogen flow is the first to set. Turn on the hydrogen, wait a minute or so for air to be purged, and set the correct flow rate, following the adjustment instructions in the manual.
Next, set the makeup flow, if used. Turn off the hydrogen flow and then turn on, measure, and adjust the makeup flow. If the hydrogen cannot be turned off conveniently then subtract the measured hydrogen flow to find the makeup flow rate. Be careful, however, when using an electronic flowmeter. If your meter has settings to select the type of gas being measured, then it will produce inaccurate readings for gas mixtures. This is not a problem for a simple soap-bubble flowmeter, nor for electronic meters that measure the volumetic flow rate.
Third, set the air flow rate. This may require a larger-volume flowmeter to accurately measure the 10-fold higher flow. Again, it is best to turn off the hydrogen and makeup flows, but you can correct the measured air flow rate if necessary.
Finally, if it’s not already on then set the carrier-gas flow. If you want to measure the carrier-gas flow rate directly at the detector then turn off the air, makeup, and hydrogen flows. Adjust the carrier-gas flow controller, pressure regulator, or EPC system as required. After column flow is established, and not before, the inlet, column, and detector may be heated to their operating temperatures.
Accurate direct measurement of capillary-column flow under about 5 mL/min requires a suitable low volume flow measuring device. For an EPC system, remember that the type of carrier gas and the column dimensions must be entered by the operator. If the entered dimensions don’t accurately reflect the actual dimensions then column flow and velocity errors will result.
Ignition
While the instrument is heating up, turn the combustion gas and makeup flows back on if necessary. You can ignite the flame as soon as the detector temperature has passed 150 °C. Most flame ionization detectors require that the air flow be temporarily reduced during ignition. Like a choke on an automobile, this reduced air flow creates a momentarily rich mixture that is easier to ignite. Some instruments have built-in igniters that are operated by push-button or from the keypad, while others have manual igniters that must be held over the detector as an internal glow wire is heated electrically. Some rely on a piezoelectric igniter. In any case, ignition is most often accompanied by an audible “pop.” EPC-equipped GC systems will automatically reduce the air flow and turn off the makeup flow during flame ignition.
Caution
Do not lean over the detector to see the flame (it is invisible), and always wear appropriate eye protection. Do not allow any clothing or other flammables to come near the detector exit.
After the flame appears to have been ignited, check for combustion water vapor by holding a cold, shiny object such as a mirror or the polished end of a wrench directly over the detector exit-you should observe “steam” condensing on the cold surface. If you do not, the flame probably hasn’t ignited or has gone out immediately. Many GC systems will report the FID background signal level as an indication of the presence of a flame. The minimum signal level is settable, but the default is usually suitable.
Flame ignition problems have several causes. Foremost is an incorrect flow setting, or possibly you forgot to turn on one of the flows. With some configurations it may be necessary to use a bakeout or startup GC method that sets the hydrogen flow rate above optimum for an even richer ignition mixture. Be sure the analytical method has the correct hydrogen flow.
Make sure that all flows are correct and that the gases are connected correctly at the back of the instrument. Flame ionization detectors will produce a very loud “pop” on ignition if the hydrogen and air lines are reversed, but usually the flame will go out immediately. Be very cautious because a large, invisible hydrogen flame that extends several centimeters above the detector may result from reversed connections.
Continued ignition difficulty may be caused by a defective igniter or other hardware problem. To check a built-in igniter, first turn off the hydrogen flow. Then press the igniter button while indirectly observing the inside of the detector with a small angled inspection mirror. For a manual igniter, observe the internal element; you should see an orange glow, or with a piezoelectric igniter you’ll see the spark. If not, then check the igniter connections and replace the igniter element if needed.
Other hardware problems that cause difficult ignition include a broken or cracked flame jet, poor detector or column installation causing leaks around the detector body, or a poorly fitting flow-measurement adapter plug that gives inaccurate flow measurements. If the detector had been operating well and then suddenly quit, check for a blocked jet tip by measuring the hydrogen flow. If required, replace or remove and clean out the jet carefully with a cleaning wire, following the manufacturer’s maintenance procedures.
It is possible to insert a capillary column through the flame jet. This will cause a large detector background signal, poor sensitivity, and usually some difficulty igniting the flame. If ignition does occur, the tip of the column can be observed-with a mirror-glowing brightly in the flame. Establish the correct ferrule-to-tip distance when installing a column.
Sometimes the flame may blow out just after injection; the solvent peak may be large enough to interrupt the flame. If this occurs often, change to a flame jet with a larger internal diameter if possible, and adjust the hydrogen flow to more closely match the carrier flow rate, being mindful of a possible sensitivity compromise. If the problem persists, you should try reducing the amount injected, using a lower carrier-gas flow rate, or both. If using a 0.53- or 0.75-mm i.d. capillary column, the problem may be caused by the proximity of the column exit to the flame jet. It may be helpful to withdraw the column somewhat or install a deactivated detector-column adapter.
Routine Troubleshooting
The flame ionization detector generally is reliable after it is properly set up. Operators can check a few key areas immediately when previously good detector performance falls below the minimum required for the application. FID is subject to two broad trouble categories: contamination and electronics. Of these, contamination is by far the more common.
Contamination
Everything that passes through a flame ionization detector is burned in the hydrogen flame. For carbon-based substances within normal levels, carbon dioxide and water are formed. Large amounts of chlorinated compounds or carbon disulfide, however, are not burned as efficiently as hydrocarbons. These materials can produce significant quantities of carbon particles (soot) as well as hydrogen chloride in the case of chloromethanes and carbon tetrachloride. Carbon particles tend to aggregate between the jet and the collector, forming an electrical leakage path, and the result is a high, noisy baseline. Hydrogen chloride from chlorinated solvents can be tolerated in small quantities, but after extended exposure in combination with the water of combustion hydrogen chloride may corrode the detector’s inner surfaces, producing electrical leakage paths and a high, noisy baseline.
Another common contamination source is stationary-phase bleed from the column. Although this is not generally a problem for most capillary columns, packed columns as well as thick film capillaries can emit significant amounts of stationary phase, especially at elevated temperatures. Siloxane polymers produce silicon dioxide when burned in a hydrogen flame. In a flame ionization detector these silica particles tend to adhere strongly to the jet and collector surfaces. These, in turn, can reduce sensitivity and increase the background signal level.
To check for detector contamination, shut off the combustion-gas flows and turn off the power to the instrument. After the instrument has cooled sufficiently, remove the detector covers and examine the outside of the detector body near the exit. It should be clean and completely free of deposits. Look down into the detector. Again, the surfaces should be clean and free of deposits. If you observe some material inside the detector, remove the collector electrode for a closer look. A black deposit indicates carbon formation. White or gray deposits are typical of silicon dioxide contamination, and green or blue-green deposits or corroded areas are a sign of excessive acid formation.
Light deposits of silicon dioxide or carbon can usually be removed from the collector by gentle scrubbing with distilled water and surfactants or in an ultrasonic bath. Be sure to first remove the collector electrode from any attached electrical connections. Ceramic insulators can also be cleaned in this manner. In general, follow the manufacturer’s recommended maintenance procedures. Detector parts that have been corroded should be replaced because cleaning is ineffective.
When the detector is reassembled, be sure that internal connections for the polarizing voltage or the collector electrode are secure. Electrical contacts can be cleaned by gently wiping them with a clean pencil eraser. Do not use any abrasives or emery cloth on detector parts--you will cause more harm than good.
Electronic Problems
FID produces minute picoampere currents. The electrometer-amplifier circuit is thus very sensitive. Although modern amplifiers and power supplies are very reliable, they do fail occasionally. Often, however, what appears to be an electronic problem is actually because of operator error. Check all instrument settings and external connections before assuming that the problem is electronic. Detector electronic and thermal parts should be tested or replaced only by a trained service technician. If the detector does not heat or the instrument reports that the temperature sensor is defective, you should not attempt to fix the problem yourself. Call in a qualified technician.
Summary
The flame ionization detector is the most familiar and widely used GC detector. It provides high sensitivity to a wide range of compounds as well as reliable routine operation. Common FID problems are few and easily identified. However, it is very important to remember that a gas chromatograph is a system that relies on the proper functioning of all its components. A problem that appears to be detector-related may in fact originate elsewhere. Perform at least a brief check of all related instrument components before concluding that the detector is at fault.
References
(1) L.S. Ettre, LCGC North Am.20, 48–60 (2002).
(2) L.S. Ettre, Chapters in the Evolution of Chromatography (Imperial College Press, London, 2008), pp. 303–320.
(3) R.A. Dewar, J. Chromatog. 6, 312–323 (1961).
(4) J.V. Hinshaw, LCGC North Am.29(1), 36–43 (2011).
(5) J.V. Hinshaw, LCGC North Am.26(11), 1100–1108 (2008).

John V. Hinshaw
“GC Connections” editor John V. Hinshaw is a Senior Scientist at Serveron Corporation in Beaverton, Oregon, and a member of LCGC’s editorial advisory board. Direct correspondence about this column to the author via e-mail: lcgcedit@lcgcmag.com
For more information on this topic, please visit www.chromatographyonline.com/column-gc-connections

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












