Challenges in the Determination of Protein Aggregates, Part II
LCGC North America
Here in part II of our series on assessing protein aggregation, we provide an overview of best practices for achieving this goal, including the importance of using a multimethod approach.Here in part II of our series on assessing protein aggregation, we provide an overview of best practices for achieving this goal, including the importance of using a multimethod approach.
Here in part II of our series on assessing protein aggregation, we provide an overview of best practices for achieving this goal, including the importance of using a multimethod approach.
In offering our thoughts on the best ways to assess aggregation in a protein sample (in terms of total weight percent aggregation and aggregate size distribution), we would like to start by defining the scope of the discussion. First, we are primarily focused on the development of biopharmaceutical (protein) drugs. Nevertheless, many of the key points mentioned here can be applied to the analysis of other biopolymers or even organic polymers. Second, we will be most focused on assessing the aggregation of a biopharmaceutical in its final stages of production-as a drug substance, drug product, or drug dosing solution that will be administered to the patient. In limiting our interest to these types of samples, we are typically dealing with aggregation levels that rarely exceed a few weight percent (wt %). Finally, we would like to consider the basic physicochemical properties of the aggregates related predominately to their intrinsic stability, which can significantly influence how one should best go about assessing them. This latter point of discussion will specifically influence a fair amount of our discussion in this article, since aggregates can dissociate into monomers or undergo further aggregation to form even higher molecular weight (MW) aggregates (which in some cases, can achieve sizes that are so big that they can precipitate out of solution). To delve more deeply into this phenomenon, we will use Figure 1.
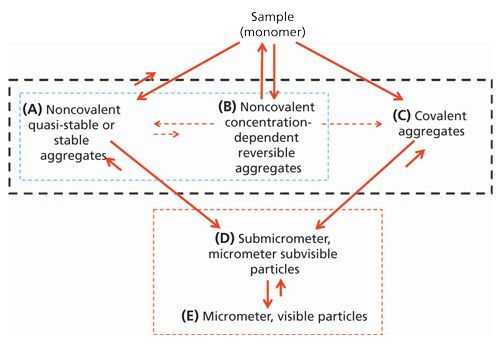
In Figure 1, we summarize the most basic physicochemical properties of biopharmaceutical aggregates and categorize them into three general classes, A, B, and C, as indicated by the area enclosed by the black dashed box. Two of these classes fall under the heading of noncovalent aggregates (A and B, as indicated by the area enclosed by the blue dashed box), while the third class (C) is characterized as covalent aggregates. The aggregates present in any of these three classes can be formed by one or more mechanisms (1). In addition, the sum of aggregates in a given biopharmaceutical sample can also be a composite of these three classes. As a result, biopharmaceutical aggregates can be very complex, heterogeneous mixtures of molecular species (2–5).
The Physicochemical Properties of AggregatesDissociation
For covalent aggregates (C), dissociation does not present significant analytical challenges, because of their robust stability. In the case of noncovalent aggregates, however, a far greater challenge is encountered. These aggregates have much more greater instability, enabling them to dissociate all the way back to monomeric species in response to changes in a sample’s physicochemical environment. In the case of biopharmaceuticals that display reversible self-association (RSA) aggregation (B), this sensitivity to environmental change can be very dramatic and induced by the simplest of sample handling operations-such as by sample dilution using the protein sample’s matrix buffer (formulation buffer) (6).
Further Self-Association
In terms of the potential for further self-association of aggregates, we are again faced with the problem of the influence of changes in the physicochemical environment of a biopharmaceutical sample in response to sampling processing, handling, and so on (7). In this case we are concerned with both the further aggregation of the aggregates with themselves and with the monomeric biopharmaceutical. This can occur through the same or different aggregation mechanisms (such as covalent aggregates undergoing further aggregation with other covalent aggregates or with noncovalent aggregates via a noncovalent mechanism). This behavior is of particular concern when attempting the development of highly concentrated biopharmaceuticals (100–200 mg/mL and higher). Under these conditions the very high biopharmaceutical concentration itself can induce RSA via very weak, nonspecific interactions (8,9). Since such weak aggregation effects may not be present at low (a few milligrams per milliliter) or even at moderate (around 10–30 mg/mL) biopharmaceutical concentrations, the detection of RSA is made very difficult because of the opposing effects of excluded volume and electrostatic effects (nonideality) that arise as the biopharmaceutical’s concentration increases that can easily mask the presence of this class of aggregation (10).
Formation of Very Large Aggregates
When further aggregation occurs, we may be forced to use biophysical tools that are far different from those commonly used to assess aggregates A–C. This regime concerns aggregates in groups D and E (indicated by the enclosed red, dashed box in Figure 1), which include submicrometer, micrometer, and visible particles. In this situation, the combined effects of the massive size of these aggregates, the extremely low level to which they are present (on a weight percent basis), and their enormous size polydispersity (relative to the much lower molecular weight oligomeric aggregates such as dimers and trimers) requires the use of very different analytical tools. For aggregate classes D and E, instead of measuring them in terms of weight percent, the number of aggregate particles per milliliter becomes the common unit of interest. Such information is acquired through direct particle observation and particle counting rather than by a mass concentration average approach used with the aggregates corresponding to A–C. It should be noted that by relying on the simple physical observation of their presence as a “particle,” as a mode of detection, serious questions can arise as to whether the observed physical particles really do correspond to aggregates of the biopharmaceutical, or if they are rather just extraneous particle contaminate material. Nevertheless, some of the newer particle analysis methodologies offer some capability to distinguish extraneous particle material from the actual aggregates of the biopharmaceutical (for example, silicon oil droplets can be distinguished on the basis of spherical shape and buoyancy or spectral analysis can help distinguish proteinaceous from nonproteinaceous particles). Such capability is an important attribute of these analytical tools in enabling them to make a more valid assessment of the presence of these classes of aggregated material in a biopharmaceutical sample (11–13).
In the remaining part of this installment, we focus on what might constitute the best approach or approaches for assessing information on the aggregates shown in Figure 1 that are highlighted by the black dashed area. In so doing we also briefly touch upon those aggregates associated with the red dashed area in Figure 1.
Methods that Involve Minimal or No Sample Perturbation
Because biopharmaceutical aggregates span an enormous range of sizes with different physicochemical properties, the best approach to accurately assess aggregation is to use an array of orthogonal techniques. In addition, it would be best if these analytical tools could minimize sample perturbation. Therefore, techniques that do not involve a sample preparation or separation step would be ideal.
The only techniques in principle that approach this ideal of not or minimally perturbing the aggregation state in a sample include static light scattering (SLS), dynamic light scattering (DLS), and to some extent analytical ultracentrifugation (AUC). In general, the assessment of aggregation using these techniques is typically carried out in the biopharmaceutical’s formulation buffer. Using these techniques, the only perturbation effect a biopharmaceutical sample will commonly encounter concerns the sample’s concentration. This alteration in concentration is commonly required to monitor the aggregation present under two different ranges of concentration: dilute concentrations (about 1 mg/mL or less) that are at or very near ideal solution conditions (which closely model the solution properties of a biopharmaceutical at zero concentration) and concentrations that range from dilute concentrations to concentrations approaching or reaching the actual concentration of the biopharmaceutical sample in its initial container closure. These two concentration ranges are needed to assess accurate information about stable aggregation (A and C) and potential aggregation as a function of increasing biopharmaceutical concentration (B), respectively.
Static Light Scattering
In the case of SLS, the assessment of aggregates in a biopharmaceutical sample is qualitatively made by measuring a biopharmaceutical’s solution MW under dilute concentration. At low enough concentration, the experimentally measured MW by SLS (which is a weight-average MW, Mw) should be effectively equivalent to the known monomeric MW of the biopharmaceutical (obtained from mass spectrometry or its amino acid sequence along with appropriate accounting of any significant post-translational modifications [PTMs]). Good agreement between these two MW values is a strong indication of no or low aggregation. Additional investigational aggregation work, but much less frequently carried out, will also involve the assessment of an increase in MW with concentration, which if present would support the presence of RSA (B) properties in the biopharmaceutical (under the experimental conditions studied).
Measuring the solution MW of a biopharmaceutical sample (using SLS) cannot provide information about its actual total weight percent aggregation level or size distribution, because there are an infinite number of aggregate size distributions that can yield any given MW value (14). However, when dealing with an initially monodisperse biopharmaceutical that displays RSA aggregation, one could attempt to quantify the aggregate levels present by identifying the correct self-association aggregation scheme that adequately models the increase in MW as a function of concentration (15,16). Nevertheless, if the RSA is very weak, aggregation of any significance will only occur at very high concentrations, where opposing, nonideality effects will make such attempts very challenging (17). Irrespective of this difficulty, a great deal of effort is presently being focused on assessing RSA aggregation and its potential adverse effect, because of the strong push in the biopharmaceutical industry to develop very high concentration, subcutaneous deliverable, biopharmaceutical products (6).
Dynamic Light Scattering
DLS offers additional capabilities for assessing aggregation in a biopharmaceutical sample. With DLS, one can measure the translational diffusion coefficient of the biopharmaceutical molecules using the same two concentration ranges discussed for SLS. This approach can provide greater information relative to SLS, by analyzing DLS data to obtain an average (z-average) diffusion coefficient, along with a polydispersity index (PDI), using what is called the cumulant method (16,18,19). Although a diffusion coefficient, like MW, does not really provide much aggregation information about aggregation, the PDI does, but it provides only qualitative information.
Another approach in DLS data analysis is to deconvolute the acquired DLS data in terms of a sum of exponentials (wherein each exponential is characterized by a unique set of autocorrelation coefficients specific to a unique homogeneous size specie present in the sample) that adequately models the experimental data. This is a much more complex process, involving the use of a non-negative least squares analysis, which incorporates fitting constraints via regularization (16,20–22). This approach can yield size distribution information about the different species of aggregates that may be present in the sample. Although this approach is useful, especially for detecting very large aggregates (like those in groups D and E), given DLS’s low resolution and high bias to the presence of very large aggregates (see the next section for further discussions of this), the size distribution information that this technique generates is more semiquantitative or qualitative in nature; weight percent compositional information about monomer, dimer, and other oligomeric aggregates present in the sample cannot be reliably extracted from this data (22,23).
Factors to Consider When Using SLS or DLS
When employing SLS or DLS, one must consider several factors that can have significant impact on the utility of the data acquired.
First, the experimentally measured intensity of light scattered from molecules is directly proportional to the product of the scatterer’s MW and its solution concentration (c). As a result, the intensity of light scattered from a sample is not a linear function of the concentration of the different size aggregates present, but rather is significantly biased by the presence of very high-molecular-weight materials (for example, if a 100 kDa monomer was contaminated with its dimer aggregate [200 kDa] amounting to only 2 wt % of the total sample, the Mw [which is related to the intensity of light scattered] obtained by SLS would equal 102 kDa, however, if that same 2 wt % of material was a 200,000 kDa aggregate the Mw would be 4098 kDa, see Table 8.2 in reference 24).
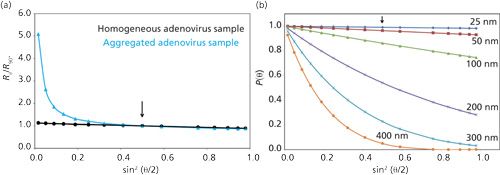
Second, the experimentally measured intensity of light scattered from molecules that have their largest dimension about 1/20 of the wavelength of the incident light or less (which will be true for virtually all monomeric biopharmaceuticals and their low-MW oligomeric aggregates-whose largest dimension is roughly about 20–25 nm or less) will be independent of the angle of observation (θ) relative to the incident beam of light (after one takes into account the normal angular dependence factors, such as the polarization state of the incident light and scattering volume). As a result, experimentally measured MW or diffusion coefficient data generated from these molecules will also be independent of θ. Consequently, under these circumstances multiangle LS (MALS) measurements provide nothing more than a statistical improvement of the data obtained over any one given angle. However, for experimental intensity LS readings on sample molecules having their largest dimensions greater than about 1/20 of the wavelength of the incident light (for example, aggregates with dimensions greater than about 20–25 nm), their intensity LS readings will show a dependence on θ. In this case, the intensity of light scattered will decrease from what it should be for a given scatterer of MW and c as θ increases (see Figure 2b). This decrease in intensity of scattered light, as a function of increasing θ and aggregate size, is caused by internal interference effects (25). Consequently, for very large scatterers, if the intensity readings are not made at low enough θ, contributions from the much higher MW material, aggregates (if present), may be reduced to very low levels relative to the noise in the data, thereby making its detection overlooked, resulting in misleading results (see Figure 2a). It is under these situations that MALS measurements are extremely useful, in detecting the presence of these very large aggregates and revealing a more accurate assessment of the amount and sizes of aggregates that are actually present.
A corollary to the second point, however, is that as θ decreases, the biggest contribution to the total light scattering readings will be dominated by the presence of the very highest MW molecules. As a result, the scattering from monomeric and relatively low-molecular-weight oligomeric aggregates can be too small to accurately detect, thereby leading to erroneous information about the true distribution of materials actually present in a sample.
As a result of the above considerations, if one is only interested in studying the lower MW soluble aggregates of classes A–C, it is recommended that one take steps to remove the LS contributions from D and E aggregates (or particulates) via clarification steps, such as centrifugation or filtration (assuming this can be done without altering the amount of lower MW soluble aggregates). In so doing, care is necessary to ensure that all containers and surfaces that will come in contact with the sample are free of extraneous, very high MW materials that might enter the sample during sample processing steps such as sample dilution, clarification, and transfer into the measurement cell. This unique issue surrounding LS measurements is generally referred to as “the dust problem,” and if not carefully controlled it can lead to confusing and erroneous results (22,24).
A potential way to circumvent the “dust problem” is to conduct LS measurements at very high θ values (for example, approaching 180°, which is referred to as back-scattering measurements). Under these conditions, the strong intraparticle interference effects of scattering from the very largest scatterers (D and E) will be reduced so their LS contributions are of little or no concern. Such an approach also allows LS measurements to be conducted at very high concentration, without encountering what is called multiple-scattering effects (scattering of the scattered light), which if not accounted for, will also result in erroneous LS data (16,26,27).
The high sensitivity of SLS and DLS to the presence of any very high MW material, coupled with the complexity of the angular dependence of these large scatterers, can have an extremely large impact, even at very low levels, on SLS and DLS measurements (see Table I). This impact, however, can make aggregation information misleading because any extraneous matter corresponding in size to class D and E aggregates (from various sources, such as containers, tubing, filters, and sampling vials used in measurements) can give rise to scattering that may not be related to biopharmaceutical aggregation, resulting in erroneous conclusions from LS data.

Regardless of these concerns and limitations, both SLS and DLS can be very useful in assessing aggregation. The quick speed with which data can be acquired in a high-throughput manner (15,23,28) and the minimal amount of sample needed to make these types of measurements make these techniques very useful in early phase biopharmaceutical candidate selection, formulation development, and in the assessment of the utility of size-exclusion chromatography (SEC) methods being used to support ongoing biopharmaceutical research and development (R&D) work (15,28–30).
Analytical Ultracentrifugation
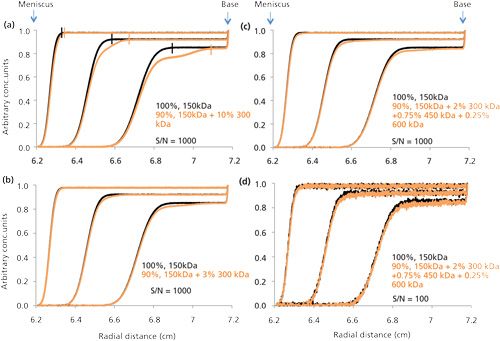
Assessing biopharmaceutical aggregation via the use of AUC (predominately using the sedimentation velocity mode of AUC [SV-AUC]) typically requires the same general approach outlined above for SLS and DLS. That is, one uses a single dilute sample and occasional series of samples of different concentrations via simple dilution with their formulation buffer. Nevertheless, in any SV-AUC experiment, the sample boundary concentration will range (after its boundary migrates clear of the sample’s meniscus) from zero concentration to values fairly close to the initial concentration of the sample loaded into the AUC cell. At the same time the sample plateau will typically only be reduced only about 20–30% from the sample’s initial load concentration during the bulk of the sample’s migration, because of the sector shape of the AUC chamber holding the sample (see Figure 3). Such changes in concentration should not affect aggregates A or C. Hence, to assess aggregation for classes A and C, all that is initially needed is to make a run on the biopharmaceutical sample at a single concentration under effectively ideal solution conditions (at a concentration of the biopharmaceutical of about 1 mg/mL or less in its formulation buffer). Results from such an experiment will generally provide adequate data that are effectively equivalent or close enough to ideal solution conditions to allow for the use of c(s) analysis via SEDFIT (31), which is the most popular SV-AUC computer software program used today. This will then deconvolute the AUC data, to yield the total aggregation and its size distribution with a reasonable level of accuracy and precision. However, when the total aggregation level of a biopharmaceutical sample falls within or below the range of about 1–3 wt %, the uncertainty in the aggregation information provided by SV-AUC via SEDFIT is not very reliable (32,33). In Figure 3a, the small black vertical bars indicate the demarcation between the two regions corresponding to the boundary located to the left of the bar, which usually goes from zero concentration to the concentration of the plateau, and the flat plateau that is to the right of the vertical bar for the data scans of the 100% 150 kDa sample. The orange vertical bar serves the same purpose for the sample with 10% 300 kDa (dimer). The progressive concentration reduction seen in the plateau height, as the monoclonal antibody (mAb) migrates from the meniscus (at 6.2 cm) toward the AUC cell base (at 7.2 cm), is because of sample dilution resulting from the required sector shape of the AUC cell’s sample chamber. Figure 3a shows the clear presence of a readily visible secondary fast moving boundary. However, at 3% aggregation (see Figure 3b) this boundary is very difficult to visualize and it is more realistically noticed by the presence of a slight sloping plateau when compared to the plateau region in the 100% mAb monomer (it is the same for Figure 3c which corresponds to the same total aggregation as in Figure 3b, but with more heterogeneous aggregated material). Figure 3d shows the enhanced difficulty in trying to quantitate this aggregation using the lower S/N UV detection, which is used in virtually all biopharmaceutical AUC work.
It should be noted that the total aggregation and size distribution information provided by SEDFIT is obtained in the same manner as that discussed for DLS (using non-negative least squares analysis). However, there are three significant differences between the deconvolution of AUC data versus the deconvolution of DLS data that make the former process more informative. The first difference concerns the nature of the mathematical form of the resulting data generated by each molecular species present in solution, which involves summing a series of exponential curves for DLS (which is known to have significant limitations in being successfully deconvoluted) versus summing a series of sigmoidal curves for SV-AUC. The second difference concerns the enhanced experimental variation of the signal output (effectively a collection of snapshots of a uniquely changing pattern of data) during the course of an SV-AUC experiment in comparison to the repetitive acquisition of the same DLS signal over time. Finally, the AUC method can better resolve or separate different sized molecular species present in a given biopharmaceutical sample by simply optimizing the gravitational field (rotor speed) (34). Overall, these differences allow for a more effective deconvolution of SV-AUC data versus DLS data because of its richer, unique, and higher resolution information content.
When using AUC to detect RSA aggregation (B), simple measurements of the weight-average sedimentation coefficient, sw, versus concentration (as carried out in terms of MW versus c in SLS or in terms of D versus c in DLS) will reveal this type of aggregation by observing an increasing value of sw as a function of the biopharmaceutical’s concentration. However, in the case of AUC, a more in-depth analysis on the RSA can be conducted (if needed)by using another powerful mode of AUC called sedimentation equilibrium (SE-AUC, which provides direct MW information independent of the samples hydrodynamic properties) in collaboration with SV-AUC analysis using more advance modes of data analysis via SEDFIT and other online available software to attempt a much deeper understanding about the aggregation and its aggregation mechanisms (35,36).
One of the greatest attributes of SV-AUC is that little or no method development is required. Samples can be simply run as is, except for some sample dilution with formulation buffer, if needed. In addition, SV-AUC can also be used to qualitatively detect the presence of aggregation at very high concentrations of biopharmaceuticals via visual observation of a sloping plateau or the appearance of more than one boundary in the sedimentation pattern traces (as shown in Figure 3), or more quantitatively using SE-AUC (32,37) in a manner similar to that outlined by Minton (38,39).
The simplicity and overall rich capability of AUC analysis in assessing aggregation is unfortunately greatly restricted from more common use in routine work that goes on in the biopharmaceutical industry, for the following key reasons.
- AUC is a low sample throughput tool. Only seven or eight samples can be looked at in a given run, which can take a day to acquire data.
- AUC has limited ability to measure the low levels of total aggregation commonly encountered in biopharmaceuticals, at or below about 1–3%.
- A high level of training and expertise is required to analyze and interpret AUC data.
- AUC instruments are expensive.
As a result, AUC analysis is restricted to tasks of a more critical nature, which include certain key experiments such as in support of investigational new drug (IND) applications and biologics license application (BLA) filings, comparability, and biosimilarity studies; SEC cross-validation work; confirmation of the presence of RSA aggregation; and in the early stages of drug research and development, at times when critical decisions need to be made, especially if uncertainty exists in the ability of an SEC method to adequately assess aggregation such as during drug candidate selection and during the early stages of initial drug formulation (6).
Methods with Potential to Introduce Significant Sample Perturbations: SEC and AF4
Two of the most attractive methods for routine aggregation assessments are SEC and asymmetric flow field-flow fractionation (AF4). When these methods are properly developed and evaluated, they are the methods of choice for assessing total aggregation and size distribution in a high sample throughput mode for all stages of drug development. Ideally, high throughput should meet the following criteria:
- Analysis can be conducted in the biopharmaceutical’s formulation buffer.
- None of the biopharmaceutical species present in the sample interact with the high surface area of the different materials present in the instrument (especially the chromatography media for SEC and the channel membrane for AF4).
- The large sample dilution required when running SEC or AF4 does not change the original aggregate species present in the sample.
- Minimal impact from the high pressures that are now being encountered with the growing use of ultrahigh-pressure SEC (40).
Unfortunately, the success of achieving the first bullet point is fairly low for SEC and only slightly better for AF4, mainly because of the difficulty associated with achieving the second bullet point. These methods are characteristically performed with a mobile phase that is different (and in some cases very different, such as when organic modifier is added to avoid sample interactions with the chromatography material) from the biopharmaceutical’s formulation buffer (41–44). Even if success is achieved in allowing the formulation buffer to be used as the mobile phase, both of these methods can require some form of column or membrane conditioning to mask binding sites that interact with the monomer, aggregates, or both. Thus, the ability to achieve the second bullet point could always linger as a potential problem that could return, as the initial binding sites reappear (because of the bleeding of the initially bound material that masks these sites) with time or the degradation of the surface properties of the chromatography material in SEC and the membrane in AF4 with the passage of time and mobile phase. Therefore, it is critical to establish an adequately representative aggregate control sample of the test drug to assess the proper working of both techniques. As a result, significant efforts are required in developing and maintaining SEC and AF4 as useful methods for assessing aggregation. In particular, it should be noted that the development efforts required for AF4 are significantly more onerous than for SEC, given AF4’s more intricate dependence on a greater number of key parameters that need to be appropriately optimized (6,44–46).
In addition, the range of concentrations that can be covered with these two methods is also significantly limited, because of the intrinsic additional sample dilution, mentioned above, that occurs during normal analysis with these techniques and the need to avoid nonideal chromatography (sample overload), which usually causes rapid loss of resolution and increased chromatographic distortion. In particular, for AF4, besides the normal dilution (which is typically more extensive than in SEC), significant concentrating is also encountered during the focusing phase of AF4. This further complicates the potential of altering and losing material during AF4. Such complexity, along with the additional challenges of optimizing a wider range of experimental parameters (44–46), tends to significantly lower the interest in developing AF4 methods (except for cases where the biopharmaceutical is at or contains some range of material that needs to be characterized that is outside the MW size range of SEC, such as viruses or virus-like particles [VLPs] [42]). This has forced many people to prefer the route of using SEC, because the development risks in SEC are lower, especially when it is supported with AUC and SLS or DLS analysis.
The introduction of various mass or size detectors, in addition to a concentration detector (for example, UV or differential refractometer detectors) to an SEC and AF4 system can significantly help in ensuring that various molecular species observed during SEC and AF4 are appropriately identified. The most notable of these other detectors that finds popular use in the biopharmaceutical industry are intensity LS detectors (via single or multiangle units) and to some extent DLS units (note, however, that the application of this latter detector to these type of measurements, which entails the dynamic movement of the sample molecules through the detector, significantly curtails the size range that can be detected).
Although these detectors do not overcome the initial potential shortcomings of SEC and AF4, the presence of an on-line SLS detector during SEC can provide an interesting approach for detecting the removal (binding, association, or disassociation) of biopharmaceutical material during SEC and AF4. This approach involves a simple comparison of the batch SLS Mw value for a biopharmaceutical sample (with and without clarification), to the Mw value obtained for the same entire injected sample in an SEC or AF4 experiment (where the LS and c values needed to calculate Mw for the entire injected sample are obtained from the appropriate integrated chromatograms generated from each detector-after accounting for any background readings using appropriate buffer blank injections). If significant differences are observed between the SEC or AF4 experiment, relative to the batch SLS experiments, one can assume this is a likely outcome of sample components retention or change in molecular size of the injected biopharmaceutical material during the actual SEC or AF4 process. Such information should be helpful in evaluating the validity of SEC or AF4 aggregation information.
Methods that Introduce Significant Sample Perturbations: SDS-PAGE (Including Capillary or Chip Gel Electrophoresis)
A common tool that falls into the category of methods that introduce significant sample perturbation is sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), which is now commonly performed in various capillary formats such as SDS-capillary or chip gel electrophoresis. Given the denaturing conditions associated with this method, the only useful aggregation data that can be extracted are those associated with covalent aggregates (C) (6). In most cases, such linkages are associated with disulfide bond formation, and to a much lesser extent involve other chemical bonds. Distinguishing disulfide linked aggregates from other covalent linked aggregates can easily be detected and distinguished by the combined application of both nonreducing and reducing SDS-PAGE. By using both, one can correlate the disappearance of high-MW bands seen on nonreducing gels relative to reducing SDS-PAGE with the presence of aggregated biopharmaceutical material held together by interchain disulfide bonds (since covalent aggregates held together by other covalent linkage would not disappear when both types of SDS-PAGE were run). Nevertheless, significant care needs to be taken in conducting nonreducing SDS-PAGE, since the presence of free sulfhydryl groups in a protein can cause disulfide crosslinking of monomer units during sample preparation heat treatment as a result of free thiol-disulfide exchange or direct coupling between free thiol groups. To avoid such an occurrence, it is common to block free sulfhydryl groups that might be present by briefly treating the sample with an alkylating agent such as N-ethylmaleimide (NEM) before heat treatment [47].
Other attributes of SDS-PAGE that could factor into its making an impact on aggregation include its ability to detect the presence of chemically broken peptide bonds along the polypeptide chain. This would be revealed by the presence of low-MW fragments that were bound to the main drug structure, by secondary forces or disulfide bonds. Such fragmented or clipped polypeptide chains could also be responsible for changes in the flexibility or conformation of the biopharmaceutical that could eventually be responsible for the formation of aggregates (especially if the low-MW fragment disassociates from the intact biopharmaceutical). Nevertheless, when characterizing multichain biopharmaceuticals that are linked by disulfide bonds into a complex monomeric structure (such as a monoclonal antibody) via nonreducing SDS-PAGE, care must be also taken to not introduce artifacts of low-MW fragment (because of disulfide scrambling as a result of the presence of free sulfhydryl groups) during the heat treatment process. Again appropriate pretreatment with NEM can minimize these artifacts (48,49).
Conclusions
In this installment, we have outlined the biophysical tools that are commonly used by biopharmaceutical scientists to assess aggregation. We have discussed these techniques in terms of what they can deliver and also in terms of their inherent limitations, which are summarized in Table I. In addition, we have also discussed the challenges that the different basic classes of aggregates present in applying these biophysical tools to extract information about them. We have also made some recommendations on how to best use these analytical tools, especially with reference to different stages in the biopharmaceutical development process.
It should be clear that the assessment of information pertaining to aggregate amounts, size, MW distributions, and properties will clearly require more than one method to minimize the risk about uncertainty in an aggregation assessment. In the early stages of drug development, however, many different variants of a biopharmaceutical candidate and experimental conditions (in early formulation development) need to be assessed and very limited amounts of material are available to study. As a result, this stage requires fast sample throughput, using as little material as possible. For assessing aggregation at this stage, an SEC method with a generic, platform or minimally optimized mobile phase is often the best choice. Nevertheless, greater certainty can be obtained by coupling SEC measurements with some batch LS measurements (which are much more robust, but qualitative in terms of their informational content) and by conducting occasional assessment, at critical junctures, with SV-AUC. At the same time, one should recognize that all separation-based methods are very suspect to causing undetected changes in the aggregation of the original sample.
As this installment has shown, there is a continuing need for better analytical methodologies to assess aggregation. We have reason to believe that this can be done. Since the call was made (50) to improve the ability to detect and quantify the subvisible and submicron aggregates found in the red-dashed boxed in Figure 1, significant progress has been made in the development of interesting and new technology (11,51,52). We can only hope the continuing call for improvement in our capability to detect and quantify the aggregates indicated in the black-dashed box in Figure 1 will also bring future improved success by way of the development of new tools or improvements to our existing tools.
References
(1) J.S. Philo and T. Arakawa, Curr. Pharma. Biotech.10, 348–351 (2009).
(2) J.S. Philo, AAPS J.8(3), E564–E571 (2006).
(3) H-C. Mahler, W. Friess, U. Grauschopf, and S. Kiese, J. Pharm. Sci.98(9), 2909–2934 (2009).
(4) J.S. Laurence and C.R. Middaugh, in Aggregation of Therapeutic Proteins, W. Wang and C. Roberts, Eds. (John Wiley & Sons, New York, 2010), pp. 1–50.
(5) R.E. Iacob, B.M. Bou-Assaf, L. Makowski, J.R. Engen, S.A. Berkowitz, and D. Houde, J. Pharm. Sci.102, 4315–4329 (2013).
(6) J.S. Philo, in Analysis of Aggregates and Particles in Protein Pharmaceuticals, H.C. Mahler and W. Jiskoot, Eds. (John Wiley & Sons, Inc., New York, 2012), pp. 305–333.
(7) W. Wang, S. Nema, and D. Tegarden, Int. J. Pharm. 390, 89–99 (2010).
(8) S.J. Shire, Z. Shahrokh, and J. Liu, J. Pharm. Sci.93(6), 1390–1402 (2004).
(9) B.D. Connolly, C. Petry, S. Yadav, B. Demeule, N. Ciaccio, J.M.R. Moore, S.J. Shire, and Y.R. Gokarn, Biophysical J.103, 69–78 (2012).
(10) N. Muramatsu and A.P. Minton, J. Mol. Recognit.1(4), 166–171 (1989).
(11) A. Hawe, S. Zolls, A. Freitag, and J.F. Carpenter, in Biophysical Characterization of Proteins in Developing Biopharmaceuticals, D.J. Damian and S.A. Berkowitz, Eds. (Elesevier, Amsterdam, 2014), pp. 261–286.
(12) S. Fischer, O. Valet, and M. Lankers, in Analysis of Aggregates and Particles in Protein Pharmaceuticals, H.C. Mahler and W. Jiskoot, Eds. (John Wiley & Sons, Inc., New York, 2012), pp. 249–267.
(13) P. Garidel, A. Herre, and W. Kliche, in Analysis of Aggregates and Particles in Protein Pharmaceuticals, H.C. Mahler and W. Jiskoot, Eds. (John Wiley & Sons, Inc., New York, 2012), pp. 269–302.
(14) S.W. Provencher, Die Makromol. Chem.180, 201–209 (1979).
(15) R. Esfandiary, D.B. Hayes, A. Parupudi, J. Casas-Finet, S. Bai, H.S. Samra, A.U. Shah, and H.A. Satish, J. Pharm. Sci.102(1), 62–72 (2013).
(16) M. Karkin and P. Wyatt, in Formulation and Process Development Strategies for Manufacturing Biopharmaceuticals, F. Jameel and S. Hershenson, Eds. (John Wiley & Sons, New York, 2010), pp. 269–305.
(17) J.S. Philo, in Methods for Structural Analysis of Protein Pharmaceuticals, W. Jiskoot and D.J.A. Crommelin, Eds. (AAPS Press, Arlington, Virginia, 2005), pp. 379–412.
(18) D.E. Koppel, J. Chem. Phys.57, 4814–4820 (1972).
(19) B.J. Frisken, Appl. Opt.40, 4087–4091 (2001).
(20) A.N. Tikhonov, Sov. Math. Dokl.4, 1624–1627 (1963).
(21) S.W. Provencher, Comput. Phys. Commun., 27, 213–227 (1982).
(22) J.S. Philo, Curr. Pharma. Biotech.10, 359–372 (2009).
(23) T. Arakawa, J.S. Philo, D. Ejima, K. Tsumoto, and F. Arisaka, BioProcessing International 5(4), 36–47 (2007).
(24) L. Makowkski, S.A. Berkowitz, and D. Houde, in Biophysical Characterization of Proteins in Developing Biopharmaceuticals, D.J. Damian and S.A. Berkowitz, Eds. (Elesevier, Amsterdam, 2014), pp. 171–209.
(25) C. Tanford, Physical Chemistry of Macromolecules (John Wiley & Sons, New York, 1961) pp. 275–316.
(26) M. Kaszuba and M.T. Connah, Part. Part. Syst. Charact.23(2), 193–196 (2006).
(27) A. Saluja, A.V. Badkar, D.L. Zeng, S. Nema, and D.S. Kalonia, Biophysical J.92, 234–244 (2007).
(28) M.L. Brader, T. Estey, S. Bai, R.W. Alston, K.K. Lucas, S. Lantz, P. Landsman, and K.M. Maloney, Molecular Pharmaceuticals12(4), 1005–1017 (2015).
(29) D. Goldberg, S.M. Bishop, A.U. Shah, and H.A. Satish, J. Pharm. Sci.100(4), 1306–1315 (2011).
(30) H.S. Samra and F. He, Molecular Pharmaceutics9, 696–707 (2012).
(31) P. Schuck, Biophys. J.78, 1606–1619 (2000).
(32) S.A. Berkowitz and J.S. Philo, in Biophysical Characterization of Proteins in Developing Biopharmaceuticals, D.J. Damian and S.A. Berkowitz, Eds. (Elesevier, Amsterdam, 2014), pp. 211–260.
(33) J.P. Gabrielson and K.K. Arthur, Methods54, 83–91 (2011).
(34) J. Dam and P. Schuck, Methods Enzymol. 384, 185–232 (2004).
(35) P. Schuck, in Protein Interactions: Biophysical Aproaches for the Study of Complex Reversible Systems, P. Schuck, Ed. (Springer Science, New York, New York, 2007), pp. 289–316.
(36) P. Schuck, in Protein Interactions: Biophysical Approaches for the Study of Complex Reversible Systems, P. Schuck, Ed. (Springer Science, New York, New York, 2007), pp. 469–518.
(37) J.S. Philo and N.M. Maluf, “New approach to Investigating the Self-Association and Colloidal Stability of Protein Pharmaceuticals at High Concentrations,” presented at the 19th Symposium on the Interface of Regulatory and Analytical Sciences for Biotechnology Health Products (WCBP 2015), Washington, DC, 2015.
(38) A.P. Minton, J. Pharm. Sci.96(12), 3466–3469 (2007).
(39) C. Fernandez and A.P. Minton, Anal. Biochem.381(2), 254–257 (2008).
(40) E.S.P. Bouvier and S.M. Koza, TrAC, Trends Anal. Chem.63, 85–94 (2014).
(41) T. Arakawa, D. Ejima, T. Li, and J.S. Philo, J. Pharm. Sci.99(4), 1674–1692 (2010).
(42) M. Wagner, S. Holzschuh, A. Traeger, A. Fahr, and U.S. Schubert, Anal. Chem. 86, 5201–5210 (2014).
(43) C. Palais, J.A. Chichester, S. Manceva, V. Yusibov, and T. Arvinte, J. Pharm. Sci.104, 612–617 (2015).
(44) U.B. Kavurt, M. Marioli, W. T. Kok, and D. Stamatialis, J. Chem. Technol. Biotechnol. 90, 11–18 (2015).
(45) T. Arakawa, J.S. Philo, D. Ejima, H. Sato, and K. Tsumoto, BioProcessing International5(10), 52–70 (2007).
(46) K.-G. Wahlund, J. Chromatography A.1287, 97–112 (2013).
(47) M.A.M. Hoffmann and P.J.J.M. van Mil, J. Agric. Food Chem.45, 2942–2948 (1997).
(48) Z.C. Zhu, Y. Chen, M.S. Ackerman, B. Wang, W. Wu, B. Li, L. Obenauer-Kutner, R. Zhao, L. Tao, P.M. Ihnat, J. Liu, R.B. Gandhi, and B. Qiu, J. Pharm. Biomed. Anal.83, 89–95 (2013).
(49) H. Liu, G. Gaza-Bulseco, C. Chumsae, and A. Newby-Kew, Biotechnol Lett.229(11), 1611–22 (2007).
(50) J.T. Carpenter, T.W. Randolph, W. Jiskoot, D.J. Crommelin, C.R. Middaugh, G. Winter, Y.X. Fan, S. Kirshner, D. Verthelyi, S. Kozlowski, K.A. Clouse, P.G. Swann, A. Rosenberg, and B. Cherney, J. Pharm. Sci. 98(4), 1201–1205 (2009).
(51) V. Filipe, A. Hawe, and W. Jiskoot, Pharm Res. 27(5), 796–810 (2010).
(52) D. Weinbuch, S. Zölls, M. Wiggenhorn, W. Friess, G. Winter, W. Jiskoot, and A. Hawe, J Pharm Sci.102(7), 2152–65 (2013).
(53) I. Krull and A.S. Rathore, LCGC North Am.33(1), 42–49 (2015).

Steven Berkowitz, PhD, is a consultant. Dr. Berkowitz received his PhD degree in Biochemistry from New York University. He then spent several years as a post-doctoral fellow at Yale University and the NIH. After his post-doctoral work, Dr. Berkowitz held various positions at Celanese Research Company, J.T. Baker, and Lederle Laboratories before taking a position at Biogen where he spent more than 20 years working in purification, analytical, and formulation development before retiring at the end of 2013.

Ira S. Krull is a Professor Emeritus with the Department of Chemistry and Chemical Biology at Northeastern
University in Boston, Massachusetts, and a member of LCGC’s editorial advisory board.

Anurag S. Rathore is a professor in the Department of Chemical Engineering at the Indian Institute of Technology in Delhi, India.
For more information on this topic, please visit www.chromatographyonline.com/column-biotechnology-today

Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.











