Fast Semivolatiles Analysis Harmonized for EPA Methods 625.1 and 8270E Using Supported Liquid Extraction and GC–MS/MS
A supported liquid extraction (SLE) and fast gas chromatography–tandem mass spectroscopy (GC–MS/MS) method, used in multiple reaction monitoring (MRM) mode, was developed for the analysis of semivolatile organic compounds (SVOCs) in environmental samples, according to the updated EPA Methods 625.1 and 8270E. This method requires minimal sample handling and yields significant throughput and productivity gains in the laboratory.
An improved, harmonized workflow within a commercial environmental laboratory is described for the analysis of semivolatile organic compounds (SVOCs) in environmental samples, according to updated EPA Methods 625.1 and 8270E, using supported liquid extraction (SLE) and fast gas chromatography–tandem mass spectroscopy (GC–MS/MS) analysis in multiple reaction monitoring (MRM) mode. Unlike liquid-liquid extraction (LLE), which has been traditionally used for Methods 625 and 8270, SLE is amenable to batch processing and decreases solvent usage while reducing labor and time. An analytical run time of 10 min for 100 targets analytes is achieved by using GC–MS/MS in MRM mode, which allows for more sensitive detection and faster batch review as a result of the elimination of matrix interferences that might be present when extracting ions in SIM or scan modes. With minimal sample handling and an added benefit of decreased instrument maintenance resulting from lower sample concentration levels, low μg/L limits are reached for up to 40 samples in less than 2 h allowing significant throughput and productivity gains in the laboratory.
Increasing sample throughput while reducing costs is an ongoing process in most commercial laboratories, and environmental laboratories often strive to modernize legacy methodologies to reach these goals. Not only do extraction methods need to be simplified and made amenable to automation, but instrumental analysis methods require updating to produce results with a faster turnaround time while maintaining a high degree of confidence. To address these needs, the EPA has updated methods 625 (625.1) and 8270 (8270E) to allow the use of solid-phase extraction (SPE) techniques to analyze for semivolatile organic compounds (SVOCs) in various sample matrices (1,4). In addition, the revised methods allow the use of state-of-the-art gas chromatography–mass spectrometry (GC–MS) instrumentation for analysis, including gas chromatography–tandem mass spectrometry (GC–MS/MS) in multiple reaction monitoring (MRM) mode.
Weck Laboratories is an accredited, full-service environmental laboratory headquartered in California. A study was undertaken here with the aim of increasing sample throughput and decreasing the costs associated with EPA Method 625.1 by converting from liquid–liquid extraction (LLE) to automated supported liquid extraction (SLE) and from GC–MS in selective ion monitoring (SIM) mode to the more selective and sensitive technique of GC–MS/MS in MRM mode. EPA Method 625, first promulgated in 1984, is used for the analysis of SVOCs in municipal and industrial wastewater. SVOCs can be classified into two subcategories of compounds known as base/neutral and acid (BNA) extractable compounds. Phenolic compounds make up the acid extractables, and polynuclear aromatic (PNA) com- pounds, or polycyclic aromatic hydrocarbons (PAHs), and phthalates are subsets of the BNA compounds (1). The method involves using LLE to extract 1 L of sample with 450 mL or more of dichloromethane. To limit the formation of emulsions, a continuous extractor using 500 mL of solvent can also be used. The extract then evaporates to a volume of 1 mL (a 1000-fold concentration of sample) and a 1-uL aliquot of the concentrate is analyzed by GC–MS. Although the LLE extraction process is efficient and simple because it requires minimal hardware, this technology has several disadvantages, including the requirement for large volumes of solvent, intensive manual labor, formation of emulsions when using a separatory funnel, large time investment for extraction (up to 24 h), and the need to evaporate the final extract. An important consideration is that the large aqueous sample volume makes this technique difficult to automate (2). At Weck Laboratories, 40 samples could be analyzed in 16 h using traditional LLE and a 30 min GC– MS-SIM analysis. This method has fairly good precision and accuracy and maximum residue limits (MRLs) of 5–10 μg/L.
The alternative and recently implemented technique of SLE, which is based on LLE principles, has been in existence for decades but has recently undergone a resurgence in popularity because of its ability to perform batch processing, obtain cleaner extracts with fewer emulsions, and decrease solvent usage.
In SLE, the same aqueous phases that are used in LLE are coated onto an inert diatomaceous earth or synthetic sorbent. The dry sorbent is contained within a cartridge, column, or a 96-well plate. The aqueous sample (which may be pH adjusted) is added and allowed to wet the sorbent bed, a process that usually takes 5–10 min. Organic solvent is then passed through the sorbent bed and a very efficient extraction takes place (3). The SLE method used in this study utilizes a synthetic medium in a cartridge format and results in two extracts for each sample (one acidic and one basic and these cannot be combined). When coupled with SLE, an analytical method using a triple quadrupole GC–MS system in MRM mode satisfied Method 625.1 criteria and resulted in MRLs of 5–10 μg/L. These detection limits are identical to those determined when using LLE sample preparation in combination with SIM analysis, but with only a 20-fold rather than 1000-fold concentration. Injecting a more dilute sample is made possible by the gain in selectivity and sensitivity through MRM data acquisition on the GC–MS/ MS instrument. An advantage is that significantly less matrix is injected into the instrument and therefore maintenance needs are reduced. A reduction in maintenance needs, therefore, leads to increased productivity. Operating in MRM mode reduces the need for a complicated batch review that may result from peak integration errors stemming from chromatographic interferences observed when using SIM or scan modes of operation. A separate analytical run is made for each of the two extracts per sample and a list of 100 target compounds can be analyzed in 10 min. Overall, the improved extraction and analysis workflow resulted in the capacity to analyze 40 samples in less than 2 h.
The same SLE protocol was applied to aqueous sample analysis according to Method 8270E, which allows for a fair amount of flexibility, so that the extraction and instrumental methods are harmonized across Methods 625.1 and 8270E (4). Method 8270 describes the analysis of SVOCs in aqueous samples, solid waste, soils, and toxicity characteristic leaching procedure (TCLP) leachates (liquid, solid, and multiphasic wastes according to Method 1311 TCLP). Method 8270E has a slightly longer target analyte list for water per California state regulations (22 CCR Appendix IX–Ground Water Monitoring List) and, because client needs dictate reporting requirements, the only difference in the workflow for the two EPA methods lies in the reporting step.
Experimental
Reagents and Materials
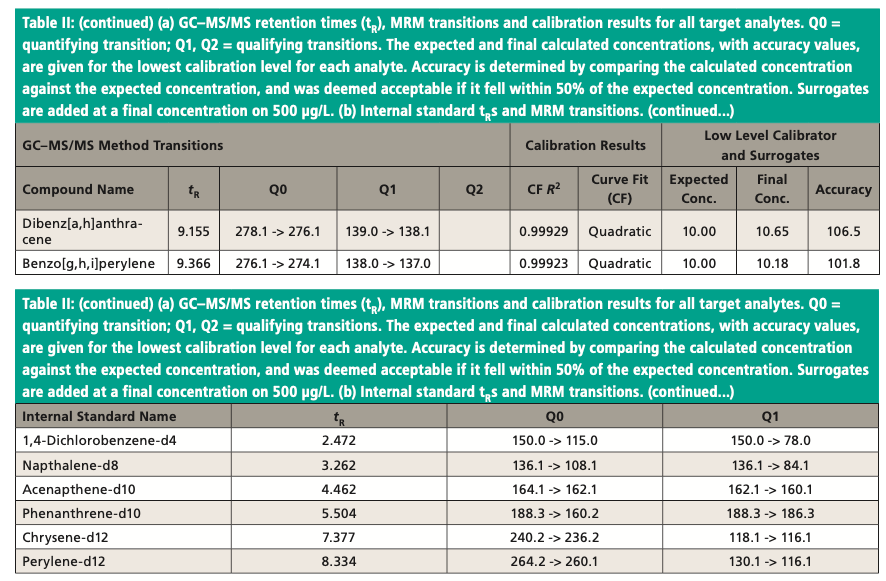
All standards were purchased from AccuStandard. Surrogate standards consisted of 2-fluorophenol, phenol-d5, nitrobenzene-d5, 2-fluorobiphenyl, 2,4,6-tribromophenol, and terphenyl-d14. Isotope-labeled internal standards used were 1,4-dichlorobenzene-d4, naphthalene-d8, acenaphthene-d10, phenanthrene-d10, chrysene-d12, and perylene-d12. Methylene chloride, reagent grade, ammonium hydroxide and formic acid were purchased from Fisher Scientific. Bulk Synthetic Chem Elut S sorbent and 60-cc, 20 mL empty cartridges were obtained from Agilent Technologies.
Sample Collection
Samples were collected in 1 L amber bottles and transported on ice to the laboratory. They were stored between 2 and 6 °C until extraction.
Procedures
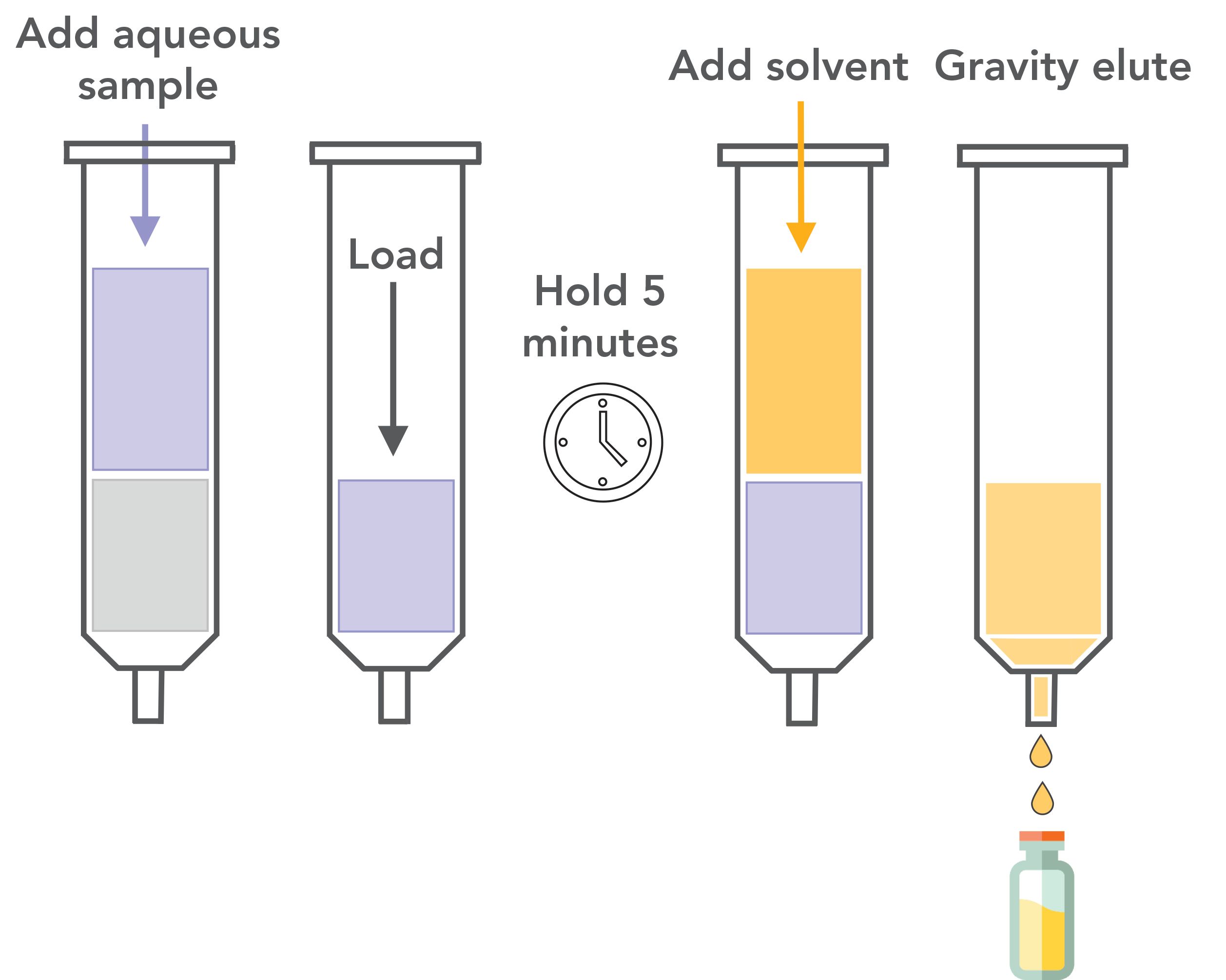
Synthetic Chem Elut S sorbent (20 g) was added to a 60-cc, 20 mL size empty cartridge, in a set of 80 cartridges to process a batch of 40 samples. Samples were processed in duplicate to collect both base/neutral and acid compound fractions. Surrogate standards were added to a 125 mL aliquot of aqueous sample for a final concentration of 25 μg/L of base/neutral and acid surrogates. An aliquot of the final concentration was adjusted to pH 12 using ammonium hydroxide to collect the acid fraction. A 20 mL aliquot of sample was loaded onto the SLE cartridge by gravity and allowed to soak for 5 min. This step was followed by the addition of 20 mL of methylene chloride and was allowed to soak for 3 min. An additional 20 mL portion of methylene chloride was then added with the first being allowed to elute by gravity before adding the second portion, which was also gravity eluted. The extracts were combined, evaporated to 1 mL, and the internal standards were added for a final concentration of 500 μg/L prior to analysis. The entire process was duplicated, using a different cartridge, with a second aliquot of the same sample adjusted to pH 2 with formic acid to collect the base/neutral fraction. This resulted in two final extracts for each sample, which were not combined. No drying step was required and the processing time for each sample was <10 min. Figure 1 shows this process.
Figure 1: Diagram showing SLE sample loading and elution.
Recoveries were determined by spiking the target compounds into reagent water at 1 mg/L and calculating their average recoveries. Due to the 20-fold concentration factor, the instrument was calibrated from 10 to 500 μg/L, which corresponded to a range of 0.5 to 25 μg/L in the sample. Statistically determined method detection limits (MDLs) using this technique were between 0.1 and 5 μg/L.
Instrumental Method
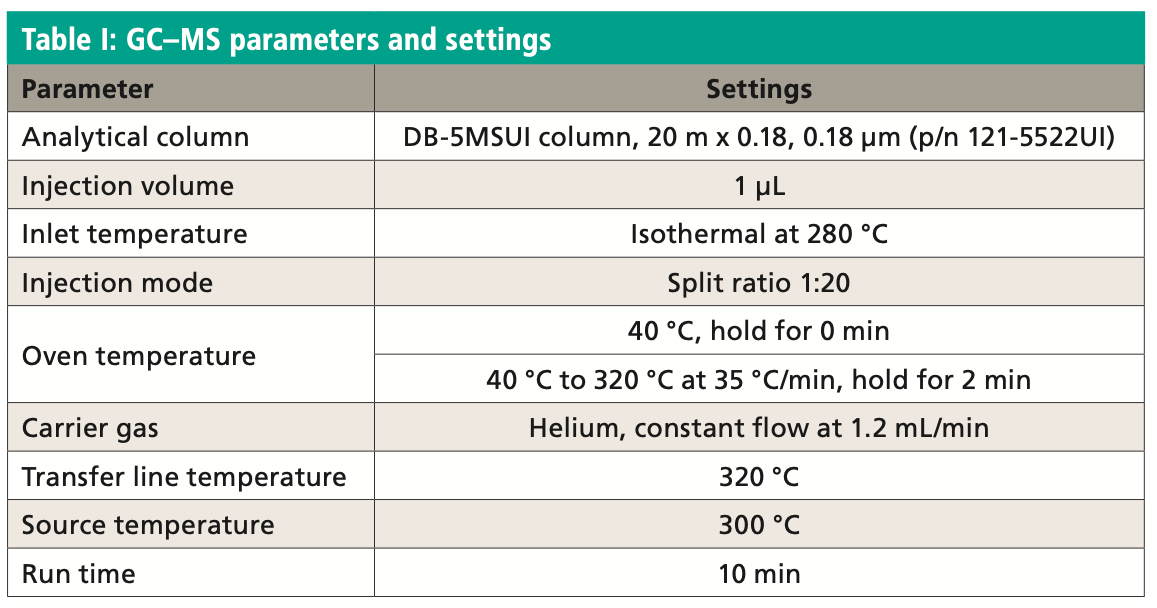
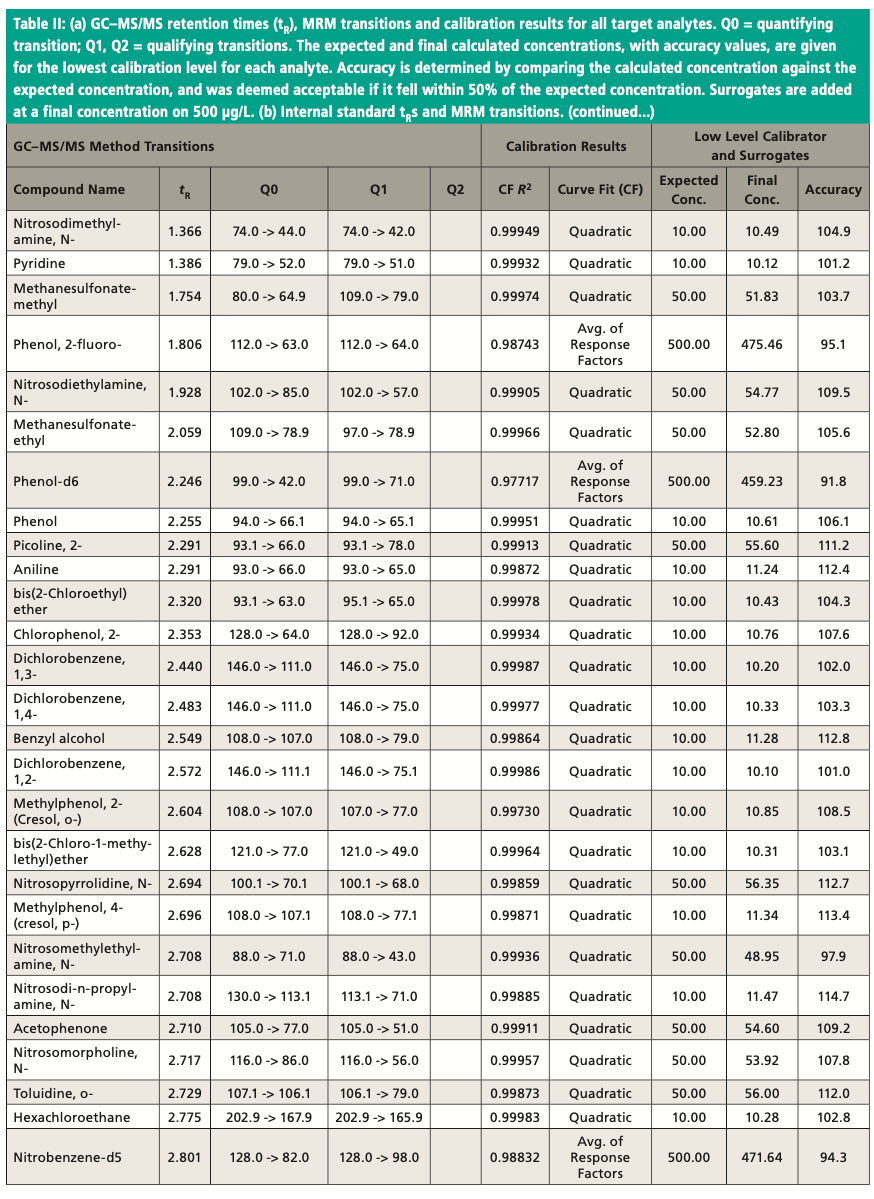
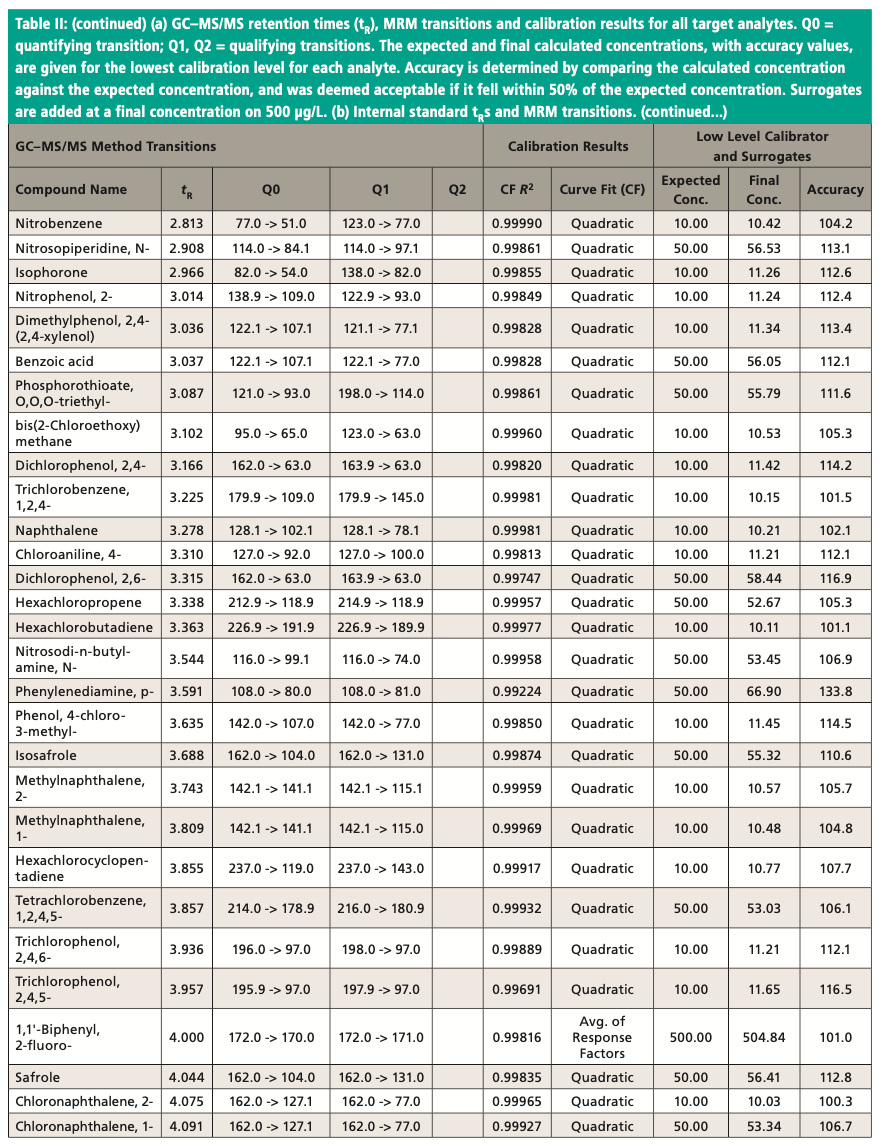
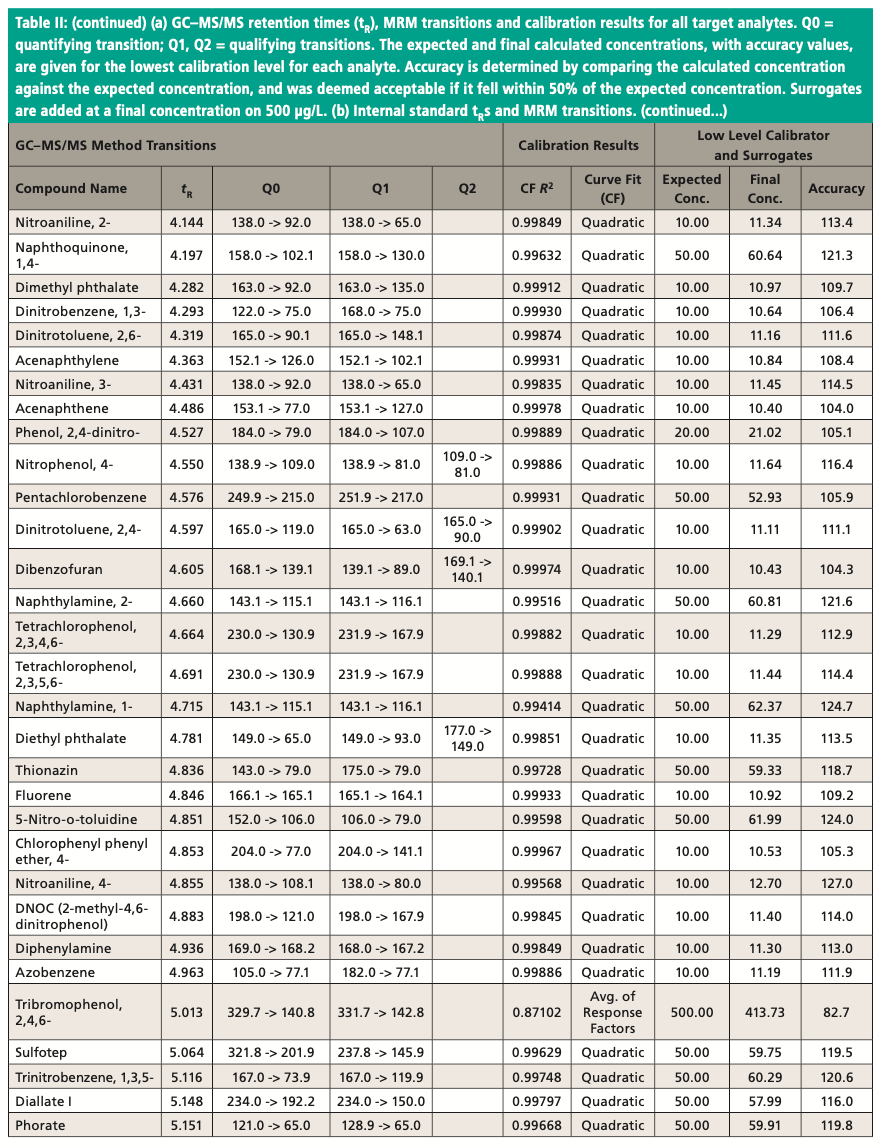
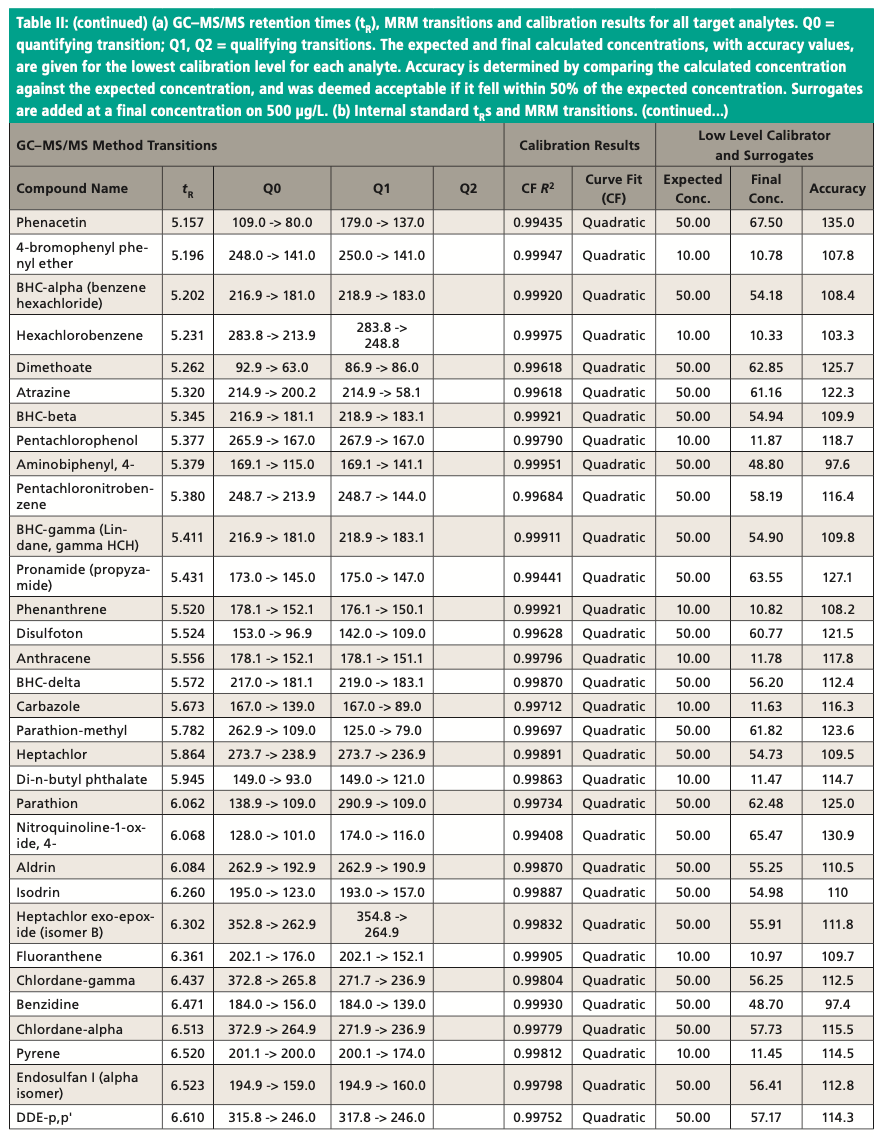
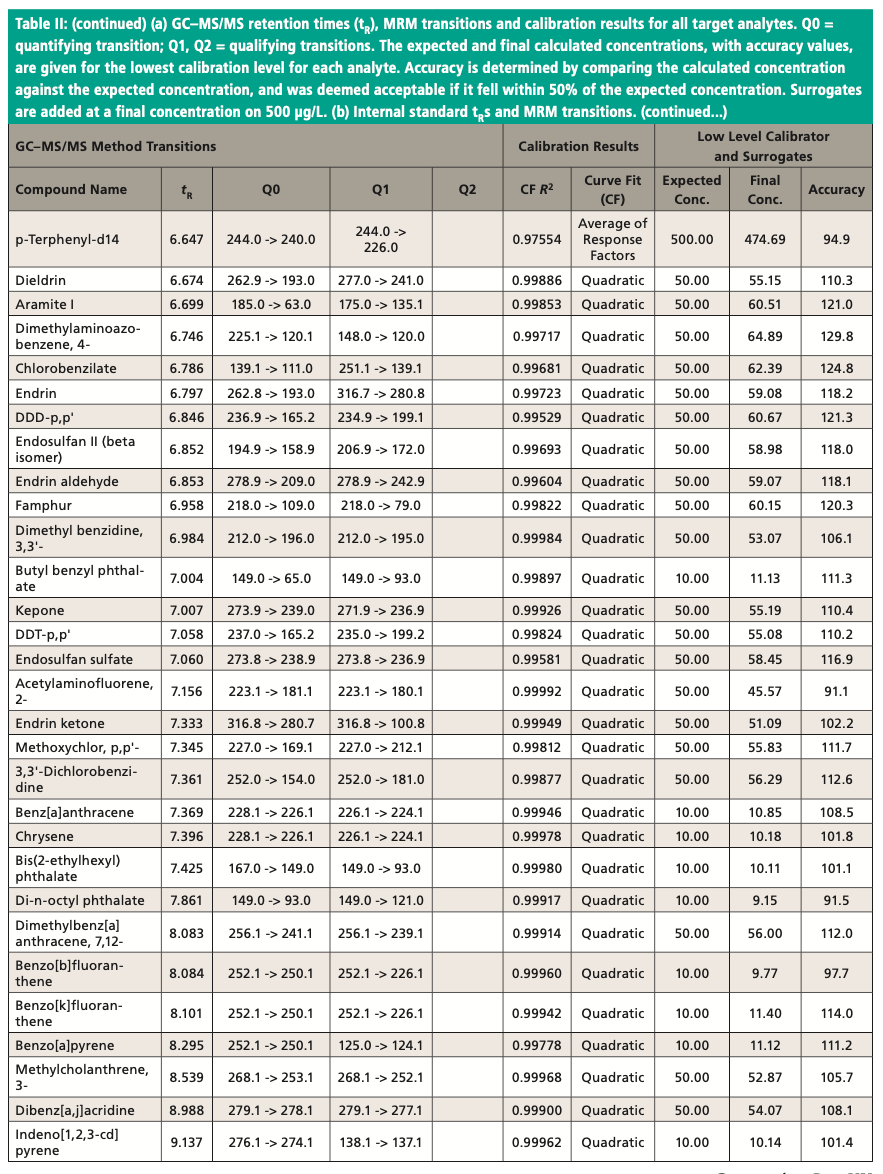
An Agilent Technologies 7010B triple quadrupole mass spectrometer was coupled to an Agilent 8890 or 7890B GC instrument with a 240 V oven for fast temperature programming. The GC instrument was equipped with an MMI inlet and an Agilent low-pressure-drop GC inlet liner with glass wool located toward the center. The system was autotuned using the autotune algorithm. Instrumental conditions are shown in Table I and compound MRM transitions are provided in Table II. The resolution settings for MS1 and MS2 (front and rear quadrupoles) were set to “unit.”
Results and Discussion
Performance of the SLE Method
The use of SLE results in cleaner extracts and fewer emulsions in comparison with LLE methods. In traditional SLE, a sorbent bed containing diatomaceous earth (DE) is used. To overcome issues associated with traditional DE sorbent, Synthetic Chem Elut S sorbent was used in this work. Unlike irregularly shaped DE particles that have considerable variability and fines, synthetic particles have a narrow particle size distribution and are free of fines. These features promote ideal flow characteristics and higher reproducibility. Also, the medium has higher sample holding capacity than DE sorbents, delivering efficient sample adsorption and reducing the chance of sample breakthrough (5). Both 2 mL prepacked cartridges, which are commercially available, and 20 mL cartridges, were evaluated. Use of the larger cartridges, which were prepared in-house using bulk sorbent, resulted in meeting the required detection limits for an expanded list of analytes. Because the final SLE protocol is based on a 20 mL sample volume, it could be easily automated for batch processing. The extraction time per sample is ~10 min, and a batch of 40 samples could be processed in 1 h by one analyst. The procedure consumes 3.2 L of methylene chloride (40 mL per sample x two extraction steps). In contrast, the time to extract 40 samples using LLE is 8 h with two analysts and requires the use of approximately 15 L of methylene chloride.
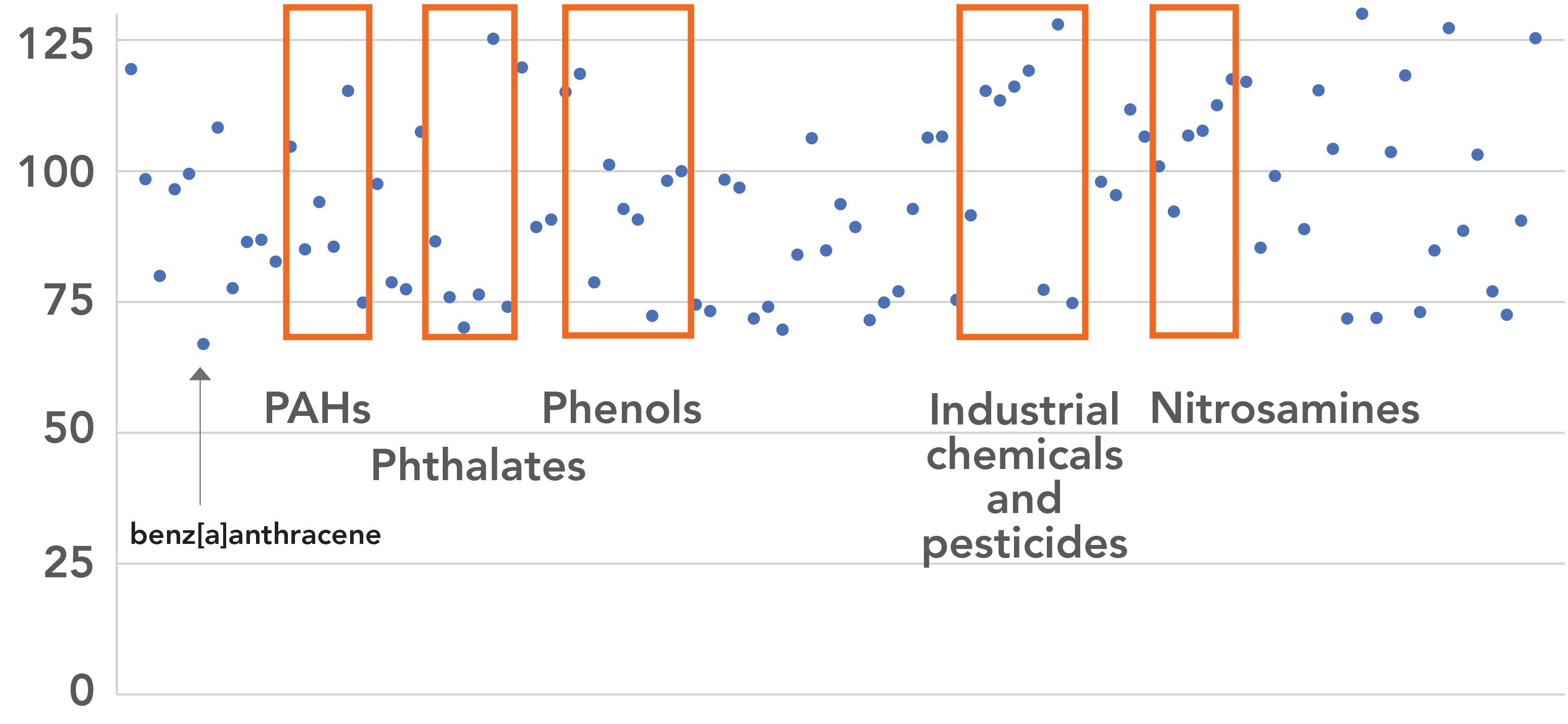
In the first phase of the work, which focused on EPA Method 625.1 only, SVOC classes of nitrosamines, pesticides, phenols, PAHs, phthalates, and PCBs in industrial discharges were analyzed. These fall into the two categories of BNA compounds and each group of compounds was extracted separately. The acid fraction containing phenols was collected first, followed by the base/neutral fraction containing PAHs, PCBs, pesticides and phthalates. Each fraction was analyzed separately. Average recoveries (n = 3) were found to be within the acceptable range of 70–130% with the exception of benzanthracene (67%) (Figure 2).
Figure 2: Average percent recovery (n = 3) for 100 target compounds at 1 mg/L is within the acceptable range of 70–130% (boxes indicate upper and lower range limits). Note: the PAH benz[a]anthracene had an average recovery of 67%.
Recoveries for an expanded list of over 150 compounds, which encompassed Method 8270, were determined as acceptable. However, a different extraction workflow was used for non-aqueous samples. Solid samples analyzed according to Method 8270 were prepared using EPA Method 3545A, pressurized-fluid extraction. The instrumentation used, more commonly known by its trade name of accelerated solvent extraction (ASE) is semiautomated (6). The extraction, which is done in a closed, pressurized vessel at elevated temperatures, uses minimal solvent and requires about 15 min per sample. Batches of up to 24 samples may be processed sequentially (7). The resulting extracts were analyzed using the same fast GC–MS/MS method.
GC–MS/MS Method
Performance and Sample Analysis
Figure 3: Chromatogram for semivolatiles list showing quantifying MRM transitions for each target analyte (75 μg/L calibration standard).
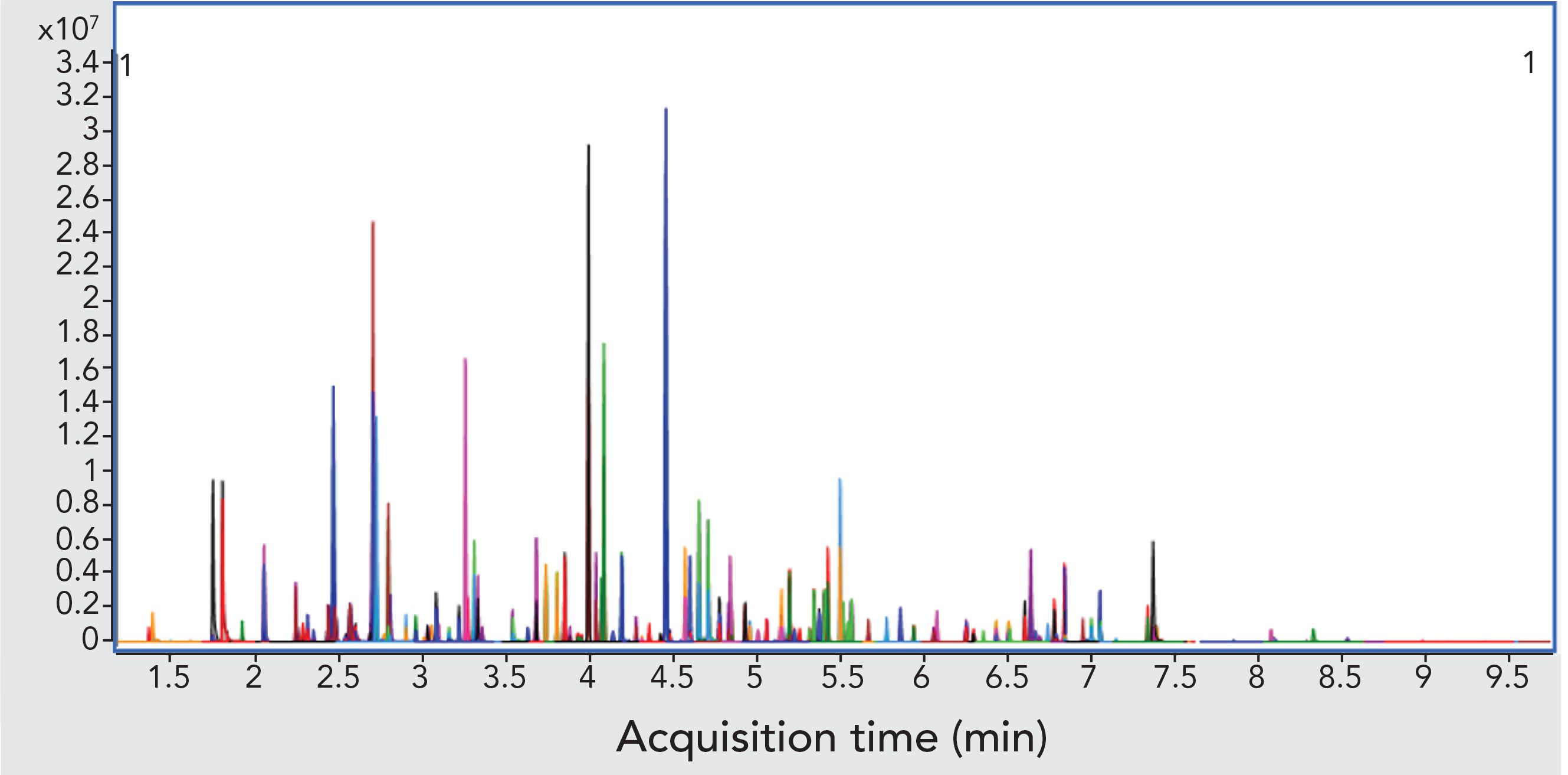
Figure 3 shows an example chromatogram for a midlevel (75 μg/L) calibration standard. Table II shows a summary of the calibration data for all target analytes and accuracy values for the lowest level in each case. Quadratic curve fits yielded correlation coefficients, R2, that were ≥0.99 and the average of response factors was used in the case of surrogates. The compound 2,4-dinitrophenol was not quantified at the lowest level but had acceptable recovery at the second-lowest level of 20 μg/L. Accuracies for the low calibrator were all well within the ±50% range established by the EPA, and all but three targets were within ±30%. All targets calibrate and behave well in the low μg/L range expected with environmental samples. Per EPA method requirements, the DDT breakdown is also monitored every 12 h.
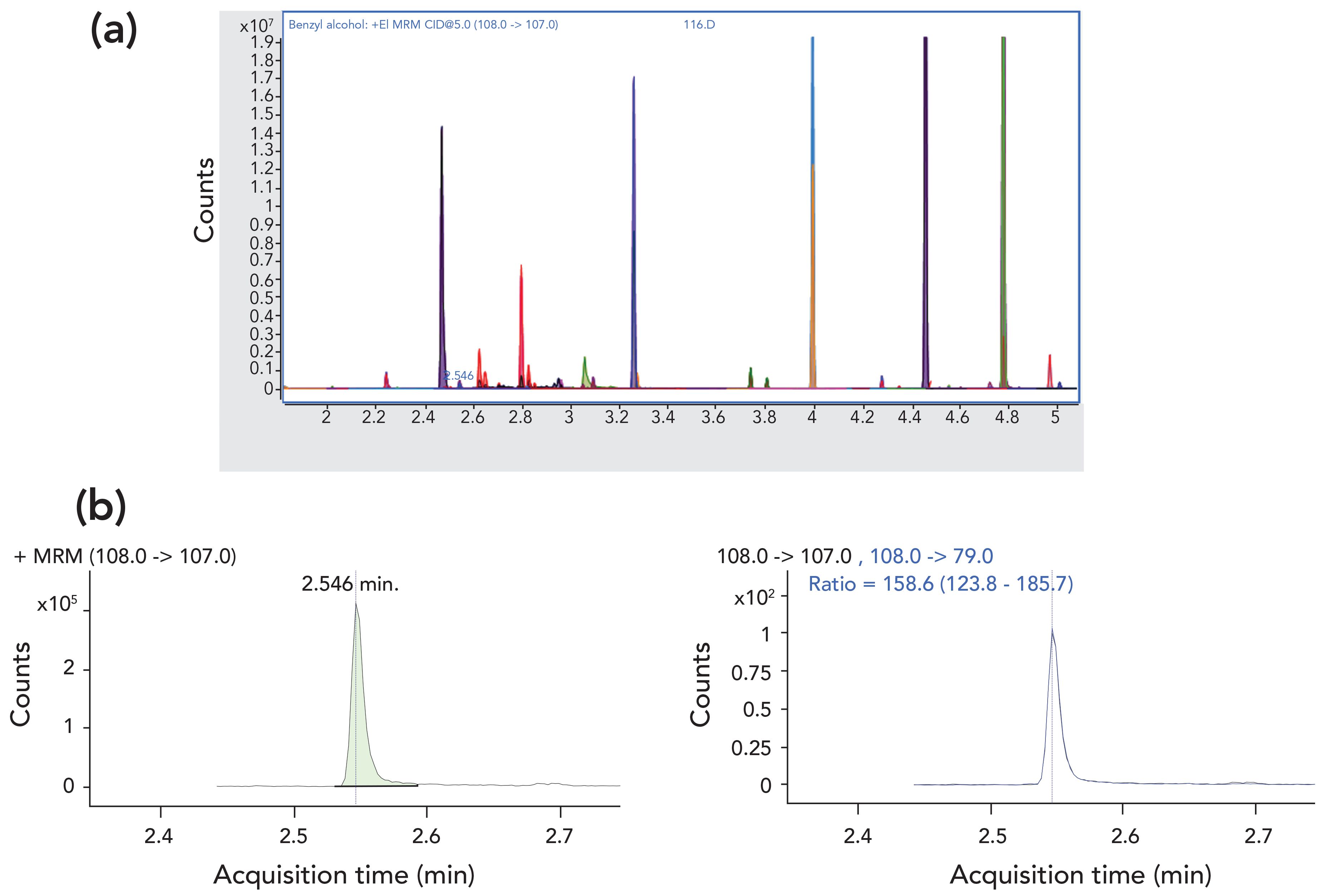
Compared to the traditional extraction and GC–MS technique utilized by the legacy methods, the approach using SLE and a fast GC–MS/MS method has several benefits. Concentrating the sample 20 instead of 1000 times results in much less matrix fouling of the instrument. This extends the inlet and column lifetime, and greatly reduces the need for frequent source cleaning. Even though each sample must be analyzed twice, the short run times and reduced downtime yield a net increase in capacity. Further increasing capacity and throughput, the specificity afforded by using MRM rather than full scan or SIM further minimizes the background matrix in complicated samples, reducing the time required for data reduction. This results in higher data quality in a shorter time window. Figure 4 shows example chromatograms for a field sample.
Figure 4: (a) Zoomed-in chromatogram for the acid fraction from a typical field water sample showing quantifying (Q0) MRM transitions for each target analyte (qualitative software package). Benzyl alcohol was eluted at 2.546 min. (b) Extracted Q0 and Q1 MRM transitions for benzyl alcohol in the same sample, shown in the quantitative software. The calculated concentration of benzyl alcohol in this sample was found to be 65.2 μg/L.
Using the benchmark of processing two batches—40 field samples with their associated quality control (QC) samples—using both techniques, the drastic cost savings are more evident. The legacy approach would take two analysts a full day of constant work to extract the samples, and they would use upwards of 17 L of methylene chloride. These two batches would then run on a GC–MS for nearly 40 h. In comparison, the same 40 samples can be extracted in roughly 1 h by the SLE approach, and both fractions analyzed in around 36 h. Besides the savings in labor costs, the SLE approach also reduces downtime and increases laboratory efficiencies. While an LLE extract must be collected in multiple flasks, combined, dried, boiled down, evaporated, and transferred to a vial, an SLE extract is simply collected directly in the concentrator tube, evaporated, and transferred to a vial. Because each sample is minimally handled by SLE when compared to LLE, there are fewer opportunities for an extract to be contaminated or lost during the extraction process, further increasing efficiency.
Using the legacy methods, field samples with highly contained matrix tend to be problematic both during the extraction and analytical portions of the method. Some samples form difficult-to-break emulsions during the extraction, extending the time and effort required for getting a clean extract for analysis. The large concentration factor from LLE also results in dirty extracts in the extract, which complicates the GC–MS analysis. The negative matrix effects are mitigated by using SLE to eliminate the potential for emulsions and extracting a much smaller sample volume. Further eliminating the potential for negative matrix effects is the use of GC–MS/MS to increase specificity and decrease the background signal during analysis.
Conclusion
Implementation of SLE with batch processing coupled with GC–MS/MS for EPA Methods 625.1 and 8270E in a commercial laboratory resulted in a significantly improved workflow. A single extraction process and analytical method were standardized for both EPA methods. A 10-min analytical run on the GC–MS System in MRM mode contributed to the ability to analyze 40 samples in <2 h of hands-on time and reach low μg/L MRLs. The use of SLE resulted in fewer emulsions, cleaner extracts, and the ability to inject less sample, which reduced instrument maintenance and led to increased instrument uptime. Simplified batch review was made possible as a result of the elimination of matrix interferences.
References
(1) U.S. EPA Method 625.1, Method 625.1: Base/Neutrals and Acids by GC–MS (U.S. Environmental Protection Agency, Washington, D.C., December 2018).
(2) J. M. Kokosa, A. Przyjazny, and M. Jeannot, Solvent Microextraction: Theory and Practice (John Wiley & Sons, Hoboken, New Jersey, 2009), pp. 4.
(3) R.E. Majors, LCGC Europe 25(8), 430–435 (2012).
(4) U.S. EPA Method 8270E, Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry (U.S. Environmental Protection Agency, Washington, D.C., June 2018).
(5) D. Lucas, Determination of Aromatic Amines Derived from Azo Colorants by GC–MS Using Supported Liquid Extraction Chem Elut S Cartridges, Agilent Applications Note 5994-0951EN (2019).
(6) G. LeBlanc, LCGC North America 19(11), 1120–1130 (2001).
Agustin Pierri and Eric Cull, are with Weck Laboratories, Inc. in Santa Barbara, California. Bruce Quimby and Melissa Churley are with Agilent Technologies, Inc. in Santa Clara, California. Direct correspondence to: Agustin.Pierri@wecklabs.com

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.
Fundamentals of Benchtop GC–MS Data Analysis and Terminology
April 5th 2025In this installment, we will review the fundamental terminology and data analysis principles in benchtop GC–MS. We will compare the three modes of analysis—full scan, extracted ion chromatograms, and selected ion monitoring—and see how each is used for quantitative and quantitative analysis.
Quantifying Microplastics in Meconium Samples Using Pyrolysis–GC-MS
March 26th 2025Using pyrolysis-gas chromatography and mass spectrometry, scientists from Fudan University and the Putuo District Center for Disease Control and Prevention detected and quantified microplastics in newborn stool samples.
The Role of SPME Combined with GC–MS for PFAS Analysis
Published: March 25th 2025 | Updated: March 25th 2025Emanuela Gionfriddo and Madison Williams from University at Buffalo, the State University of New York, NY, USA discuss the important role that solid-phase microextraction (SPME) techniques with gas chromatography mass spectrometry (GC–MS) can play in the analysis of per- and polyfluoroalkyl substances (PFAS).






