A Turnkey Protocol for Detecting Taste and Odor Compounds in Drinking and Surface Waters by Gas Chromatography–Mass Spectrometry and Electron Capture Detector (GC–MS/ECD)
A comprehensive monitoring protocol has been developed using GC–MS/ECD in selective ion monitoring (SIM) mode, with injection performed by solid-phase microextraction (SPME) and headspace (HS). This single system has been configured to analyze for all taste and odor (T&O) compounds in Standard Method 2170, with minimal changing of columns, injectors, or SPME fibers between methods.
Wichita Falls, Texas, is a surface water system that experiences routine taste and odor (T&O) events. T&O events are caused by the production of volatile and semivolatile organic compounds (VOCs and SVOCs) during harmful algal blooms (HABs) or during the breakdown of organic matter. Algae and cyanobacteria readily produce these metabolites, which can be costly to public water systems. Compounds of interest have been identified in Standard Method 2170 on the T&O wheel, and have been grouped by category based on taste, odor, and chemical structure. Unfortunately, there is no single method to analyze all compounds without time- and labor-intensive work. To mitigate this problem, the City of Wichita Falls Cypress Environmental Laboratory has designed and implemented a comprehensive monitoring protocol using a single-quadrupole gas chromatograph-mass spectrometer/electron capture detector (GC–MS/ECD) in selective ion monitoring (SIM) mode, with injection performed by solid-phase microextraction (SPME) and headspace (HS). This GC–MS/ECD system has been configured to run methods to analyze for all T&O compounds in SM 2170 likely to affect surface waters, with minimal changing of columns, injectors, or SPME fibers between methods. Testing is performed on samples from two surface water reservoirs, one holding reservoir, and two water treatment plants. After over four years of successful implementation, customer complaints have been all but eliminated as a result of the mitigation of 12 T&O events before water containing T&O compounds was discharged to the City’s residents. To date, it has been 1491 days since this strategy was implemented, with no failures. This article outlines the analytical methods used to detect each compound category on the SM 2170 T&O wheel.
The first recorded taste and odor (T&O) event in the United States was a “peculiar cucumber odor” that occurred in Boston in 1854 (1). Standard Methods for Water Analysis was first published in 1905, recognizing that T&O issues were caused by both microorganisms and algae, and stated “the odors are usually connected with some organic growths or with sewage contamination, or both” (2). Several thousand peer-reviewed works have been published on this topic since the 1940s, with the most recent guides being the T&O wheel in Standard Method 2170 and Taste and Odour in Source and Drinking Water, which includes a modified biological T&O wheel (3,4).
For decades, T&O events have been routine in Wichita Falls, Texas. All this changed during the height of a historic drought from July 7, 2014 through July 21, 2015, when the public water system (PWS) operated the world’s largest direct potable reuse (DPR) system (5). Half the daily water supply was produced from wastewater effluent to supplement dwindling surface water reservoirs. During this time, there were no noticeable T&O events because they were blended out during treatment. After record rainfall, the DPR was decommissioned and the region began experiencing regular T&O cycles again. In the fall of 2016, the region saw the worst T&O event in recent memory, and the PWS received hundreds of customer complaints. This led to the design and implementation of a T&O monitoring plan (6).
Harmful algal blooms (HABs) exemplify the tenacious ability of cyanobacteria to exploit their environment, offering clues to suggest ecosystem imbalance. The cyanobacteria blooms are typically associated with eutrophic systems, but that is not always the case. Forecasting is difficult because environmental factors (such as light, temperature, dissolved oxygen, and nutrient loading) can be similar during bloom and non-bloom periods. There is no complete catalog of metabolites produced by specific taxa, and all known T&O compounds are produced by multiple genera, so there is no true way to fingerprint a T&O compound to one taxon (7). These dynamic variables make it impractical and ineffective for a PWS to rely on a single type of analysis to manage T&O issues (8). To overcome this, the Cypress Environmental Laboratory was directed by the City of Wichita Falls to design and implement a T&O monitoring plan that merged analyses from the microbiological laboratory and the analytical (9). The laboratory incorporated a flow-imaging microscope (FlowCam) with traditional threshold odor testing and modified flavor profile analysis (SM 2150B and 2170), a new gas chromatograph–mass spectrometer/electron capture detector (GC–MS/ECD) system, and Phytoxigene’s molecular-based CyanoDTec assay using quantitative polymerase chain reaction (qPCR).
The flow-imaging microscope is used to identify and enumerate algae and cyanobacteria, and sort them into groups using semi-automated image recognition technology (such as T&O-producers and filter-cloggers) (10). Knowing which taxa are present enables the laboratory to determine whether a T&O or cyanotoxin event is likely to happen. Chemical analysis indicates a current problem already present in the water column while identifying organisms indicates that heightened monitoring is prudent even if the compounds have not been initially detected. A “low detect” or “nondetect” result may indicate that the compounds have not yet been released by T&O–producing organisms. Intracellular compounds can be present but not readily detected until they are released during normal growth or senescence. Their undetected presence does not absolve the utility of a potential problem. When a T&O event begins, it is important for quality data to be systematically collected to give guidance for effective mitigation (11).
The laboratory worked with Thermo Fisher Scientific to design a GC–MS/ ECD system that could analyze all T&O compounds likely to be found in surface waters. By merging flow-imaging microscopy and GC–MS/ECD, the laboratory could notify the water utility and proactively respond to T&O events. Additionally, because many of the region’s most problematic cyanobacteria are known to produce cyanotoxins, qPCR was included to detect the presence of cyanotoxin-producing genes in surface waters.
Both source lakes, a holding reservoir, and both water treatment plants are monitored based on a seasonal schedule (12). Warmer summer months are scheduled for monitoring 3–5 days per week, whereas colder winter months are scheduled for monitoring 1 day per week. When T&O events are detected, the utility superintendent is notified, and testing is increased. If the event continues to grow, sources are changed, and the utility will only draw water from the lake without the bloom. If blooms occur in both lakes, then treatment at the plant is increased to include chlorine dioxide as a primary disinfectant of raw water, potassium permanganate in the plant lagoons and clarifiers, and powered activated carbon (PAC) in the clarifier mix zone to adsorb and settle out T&O compounds during treatment. During this time, the laboratory increases testing and makes treatment recommendations until the bloom subsides. The goal is to achieve control at the source through active monitoring. For this to work effectively, there must be a clear understanding of the limnology of lakes and reservoirs, and the infrastructure that links all the components of a storage and conveyance system together (13). The focal point of the monitoring plan and water treatment changes are based on cyanobacterial counts by flow-imaging microscopy and the T&O compound detection by GC–MS/ECD.
Materials and Methods
Perceptions of tastes and odors in water are the result of chemical stimuli interacting with receptor cells. Almost all organic compounds can produce stimuli when in contact with receptor cells. These compounds are malodorous, but not toxic at low levels in surface water (14). Compounds are produced over the growth and senescence of a wide variety of cyanobacteria, which can lead to short-term and long-term changes in surface waters. Emerging knowledge reveals that benthic cyanobacteria can be as problematic as planktonic forms suspended in the water column. Both groups should be monitored during T&O events. The chemistry of T&O compounds is diverse, including terpenoids, sulfides, lipid and pigment derivatives, aromatics, hydrocarbons, amines, aldehydes, alcohols, and others. Threshold levels for detection in water span several orders of magnitude, depending on the type of compound, making detection of more potent compounds difficult at trace levels.
Figure 1 shows how Standard Method 2170 has grouped T&O compounds into four taste categories and eight odor categories. The descriptors loosely reflect similar chemical compositions, which can help explain why certain treatments are more effective for a particular group of odors (15,16). The compounds in the odor category are the most problematic for water systems, and this subset is the focus of this protocol.
Figure 1: The taste and odor (T&O) wheel in Standard Method 2170.
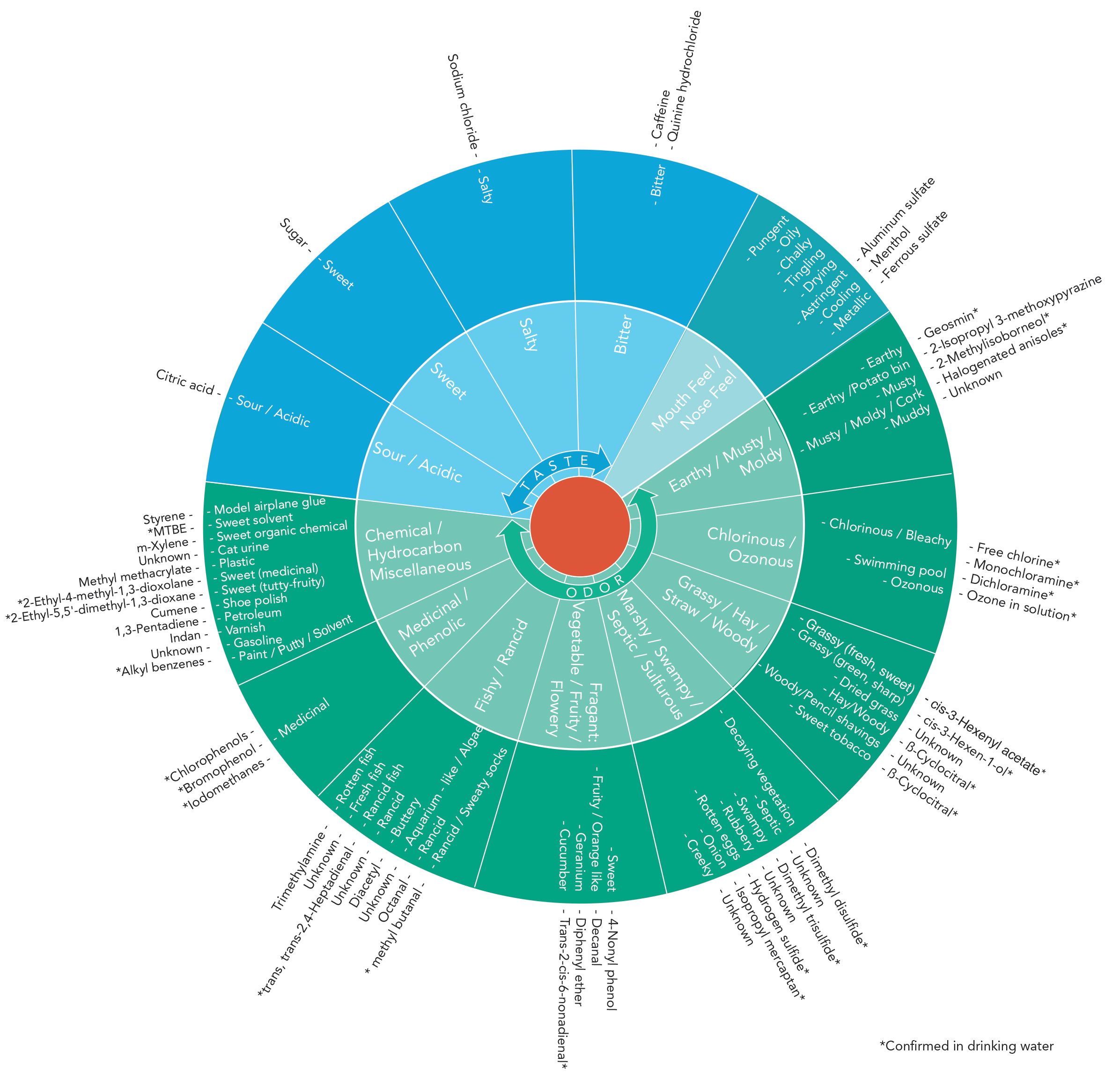
Earthy/Musty/Moldy Category
The earthy/musty/moldy category includes the most common T&O compounds that water treatment systems deal with. It includes two terpenoids, geosmin (trans-1, 10-dimethyl-trans-9-decalol), and 2-MIB (2-methyl-isoborneol). These compounds are produced by a wide variety of taxa, including bacteria (actinomyces and mxyobacteria), cyanobacteria, fungi, bryophytes, protozoans, and some plants. Geosmin and MIB are potent, chemically stable over long periods of time, and resist conventional water treatments. Also included in the earthy/musty/moldy category are 2-isopropyl 3-methyoxypyrazine (IPMP), and halogenated anisoles like 2,4,6-trichloroanisole (2,4,6-TCA).
Table I displays the compound information for the earthy/musty/moldy category. Human detection thresholds are as follows: geosmin: 6–10 ng/L; MIB: 2–20 ng/L; IPMP: less than 1 ng/L; and halogenated anisoles: less than 1 ng/L.
Analysis of this category of compounds is performed by SIM using a Thermo Scientific Trace 1310 ISQ LT GC–MS instrument with a Trace-GOLD-5MS column, and sample injection by a Supelco solid-phase microextraction (SPME) divinylbenzene/carboxen/polydimethylsiloxane (DVB/ CAR/PDMS) fiber. A Thermo Scientific TriPlus RSH autosampler is used with a three-position sample tool changer. This injection is performed by RSH sample tool 1. SIM is a mode in which a limited mass-to-charge ratio range is transmitted and detected, resulting in higher selectivity and sensitivity. Sodium chloride is added to samples and dissolved with heat to increase ionization potential and drive the VOCs and SVOCs from the aqueous phase to the gaseous phase. SPME is a sample injection method in which target compounds adsorb onto a SPME fiber in the headspace of a closed sample vial, which is then immediately inserted into the heated injection port of a split–splitless (SSL) injector. Compounds then desorb from the SPME fiber onto the GC column. After separation in the GC column, target compounds are detected in the mass spectrometer (MS) by monitoring the largest of multiple target ion masses for confirmation and quantified with the instrument software. IBMP (2-isobutyl 3-methoxypyrazine) is used as an internal standard.
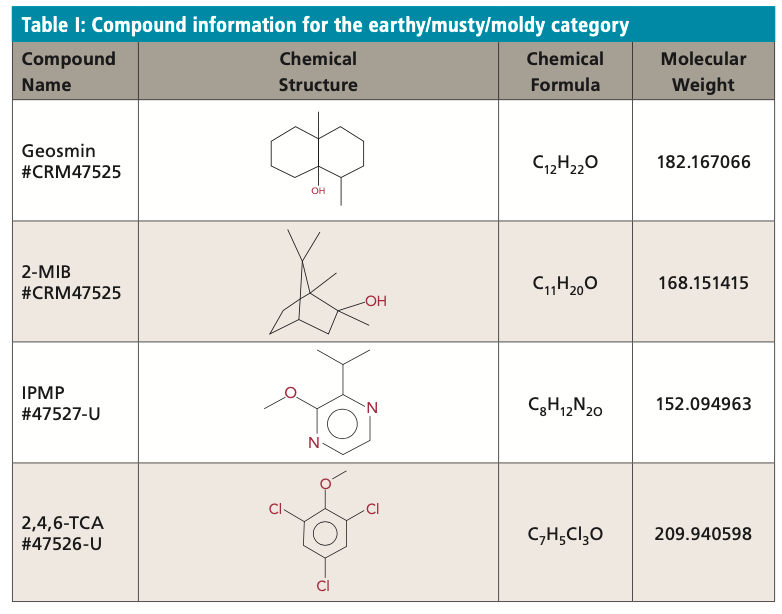
Grassy/Hay/Straw/Woody Category
The grassy/hay/straw/woody category includes semiochemicals like esters, alcohols, and fragrant apocarotenoids. The ester cis-3-hexenyl acetate is found in plants and is released in response to tissue injury as a fungicide, bactericide, or as a defense chemical. Alcohols like cis-3-hexen-1-ol are produced by plants to attract predatory insects. The apocarot-enoid β-cyclocitral is derived from the pigments carotene and xanthophyll that are widely distributed among different cyanobacteria. Production is a function of the type and abundance of these pigments and mostly occurs at cell lysis, which can be an indicator for a potential increase in cyanotoxins. The human detection thresholds are as follows: cis-3-hexenyl acetate: 70 ng/L; cis-3-hexen-1-ol: 70 μg/L; and β-cyclocitral: 20 μg/L. Table II displays all the compound information for the grassy/hay/straw/woody category.
Analysis of this category of compounds is performed by SIM using a Thermo Scientific Trace 1310 ISQ LT GC–MS instrument with a Trace-GOLD-5MS column, and sample injection by a Supelco SPME DVB/ CAR/PDMS fiber. This injection is performed by TriPlus RSH sample tool 1. Sodium chloride is added to samples and dissolved with heat. IBMP is used as an internal standard.
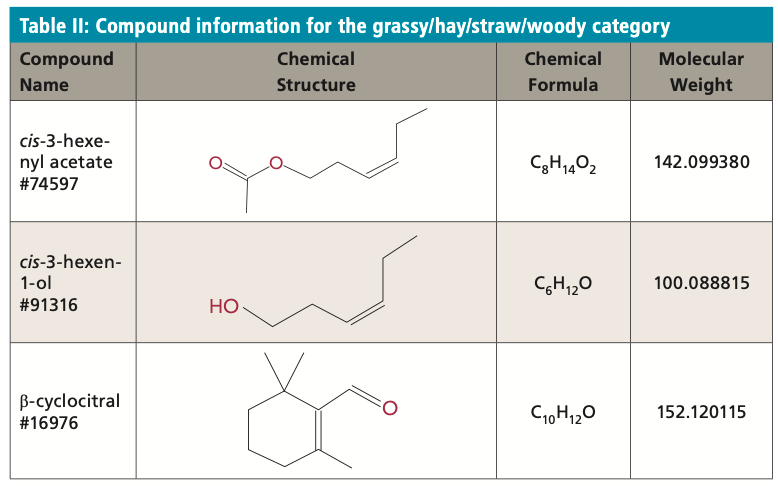
Fishy/Rancid Category
The fishy/rancid category consists of aldehydes and amines that are produced by taxa with high cellular polyunsaturated fatty acids (PUFAs). Octanal is a saturated fatty aldehyde with a fruity odor. Isovaleraldehyde is an aldehyde produced by plants with a powerful acrid odor. The aldehyde trans,trans-2,4-heptadienal is an allelopathic polyunsaturated aldehyde (PUA) that is highly bioactive, involved in diatom–copepod interactions, and resistant to treatment. Trimethylamine is produced by the reaction of ammonia with methanol and produces a strong fishy odor. The human detection thresholds are as follows: octanal is unknown; isovaleraldehyde: 150 μg/L, trans,trans-2,4-heptadienal: 5 μg/L; and trimethylamine is unknown. Table III displays the compound information for those compounds in the fishy/rancid category.
Analysis of this category of compounds is performed by SIM using a Thermo Scientific Trace 1310 ISQ LT GC–MS instrument with a Trace-GOLD-5MS column, and sample injection by a Supelco SPME DVB/ PDMS fiber. This injection was performed by TriPlus RSH sample tool 3. Sodium chloride and potassium hydrogen phthalate (KHP) are added to samples and dissolved with heat. Analysis of this category involves a long derivatization step with PFBHA (O-2,3,4,5,6-pentafluorobenzylhydroxylamine), which is performed in an RSH incubation module. This reaction produces isomers that must be integrated for quantification. 1,2-DBP (1,2-dibromopropane) is used as an internal standard.
Marshy/Swampy/Septic/Sulfurous Category
The marshy/swampy/septic/sulfurous category are rarely produced by cyanobacteria during normal conditions, but they are major constituents produced in anaerobic decomposition of HABs and other organic material. Surface waters heavily impacted by agriculture and wastewater input realize these compounds in abundance. Isopropyl thiols in this category, together with the carotenoid derivative β-cyclocitral from the grassy/hay/ straw/woody category, contribute to the distinct sulfurous, tobacco, and musty odor produced during Microcystis blooms. Dimethyl disulfide (DMS) is an oxidation product of methanethiol in air produced during bacterial decomposition. Also produced during bacterial decomposition is dimethyl trisulfide (DTS). Isopropyl mercaptan is an isopropyl thiol often found with DMS and DTS and has a strong skunk-like odor. The human detection thresholds are as follows: dimethyl disulfide: 10 ng/L; dimethyl trisulfide: 10 ng/L; and isopropyl mercaptan: less than 1 pg/L. Table IV displays the compound information of all compounds in the marshy/swampy/ septic/sulfurous category.
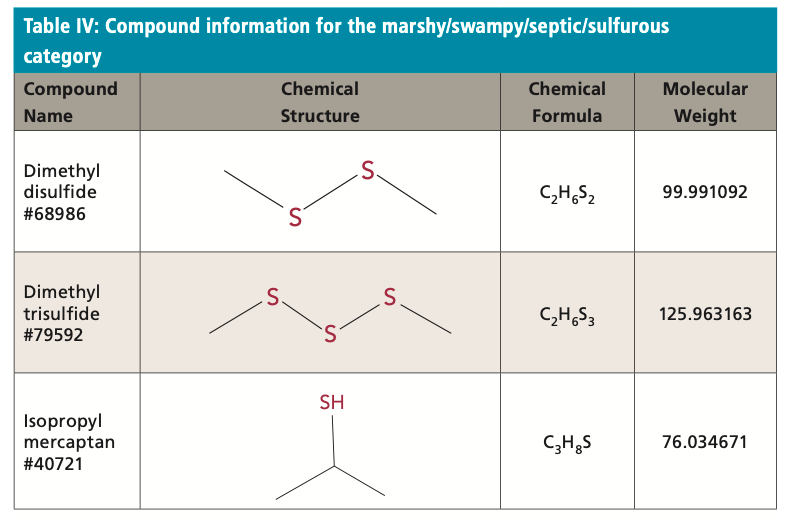
Analysis of this category of compounds is performed using a Thermo Scientific Trace 1310 GC-ECD instrument with a TraceGOLD-1MS column, with sample injection by TriPlus RSH HS. This injection is performed by RSH sample tool 2. Sodium chloride is added to samples and dissolved with heat.
Medicinal/Phenolic Category
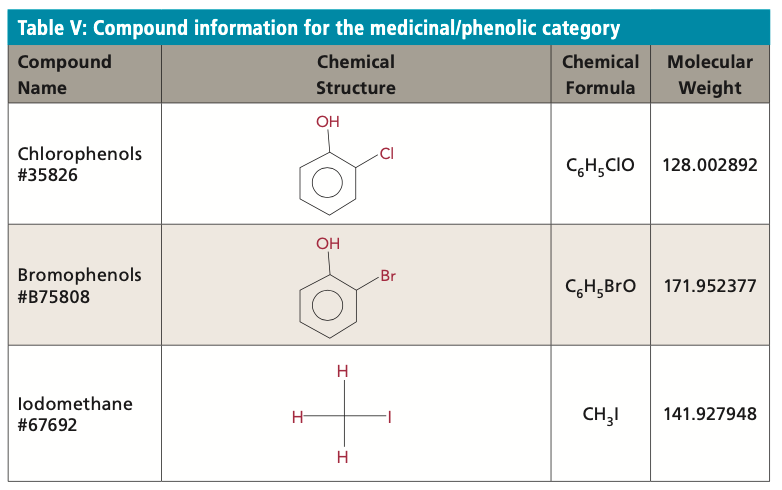
The compounds in the medicinal/phenolic category are not a common source of concern for surface water systems. This category is made up of phenols (chlorophenols and bromophenols) produced by electrophilic halogenation and methanes (iodomethane) produced by algae. The odors produced are commonly described as smelling like pesticides, herbicides, and disinfectants.
Analysis of this category of compounds is performed by SIM using a Thermo Scientific Trace 1310 ISQ LT GC–MS instrument with a Trace-GOLD-WaxMS column, and sample injection by a Supelco SPME PA (polyacrylate) fiber. This is the only category in this protocol in which analysis includes changing the SPME fiber and column. Sodium chloride is added to samples and dissolved with heat. Table V displays the compound information for those that are in the medicinal/phenolic category.
Fragrant/Vegetable/Fruity/Flowery Category
The fragrant/vegetable/fruity/flowery category is comprised of alkylphenols and aldehydes. These compounds are not regular nuisance compounds, so the authors do not actively monitor for compounds in this category. These compounds produce aromas of cucumber, bread, watermelon, grapes, tea, and vanilla. The category includes the plant metabolites decanal, diphenyl ether, trans-2-cis-6-nonadienal, and the xenobiotic, 4-nonyl phenol.
Chemical/Hydrocarbon Category
The chemical/hydrocarbon category also includes compounds not normally found in surface waters, but they can be present as a result of spills or sewage. This category includes compounds like methyl tert-butyl ether (MTBE), benzene, toluene, ethylbenzene, xylenes (BTEX), and styrene. Due to the lack of their natural presence in surface waters, they were not included in this turnkey protocol. However, they are easily monitored for by laboratories already running EPA 524.2, which outlines the detection of purgeable organics and can be performed by SIM using a Teledyne Tekmar Aqua Tek 100 autosampler and Stratum P&T (Purge & Trap) and Thermo Scientific Trace 1300 ISQ QD GC–MS instrument with a Trace TR-524 column. If laboratories already monitor for disinfection byproducts like trihalomethanes (THMs), the compounds in the chemical/hydrocarbon category can be analyzed using the same method parameters.
Chlorinous/Bleachy Category
Like the previous category, the chlorinous/bleachy category includes compounds not normally found in surface waters, so these compounds were also not included in this turn- key protocol. This class consists of constituents used as disinfectants in the treatment of surface water like free chlorine, monochloramine, dichloramine, and ozone, so it is already understood that they will be present to some degree in finished tap water. Due to the fact that they were added or formed in the disinfection process, laboratories should already have the capability to analyze for this class by colorimetric testing or amperometric titration.
Results
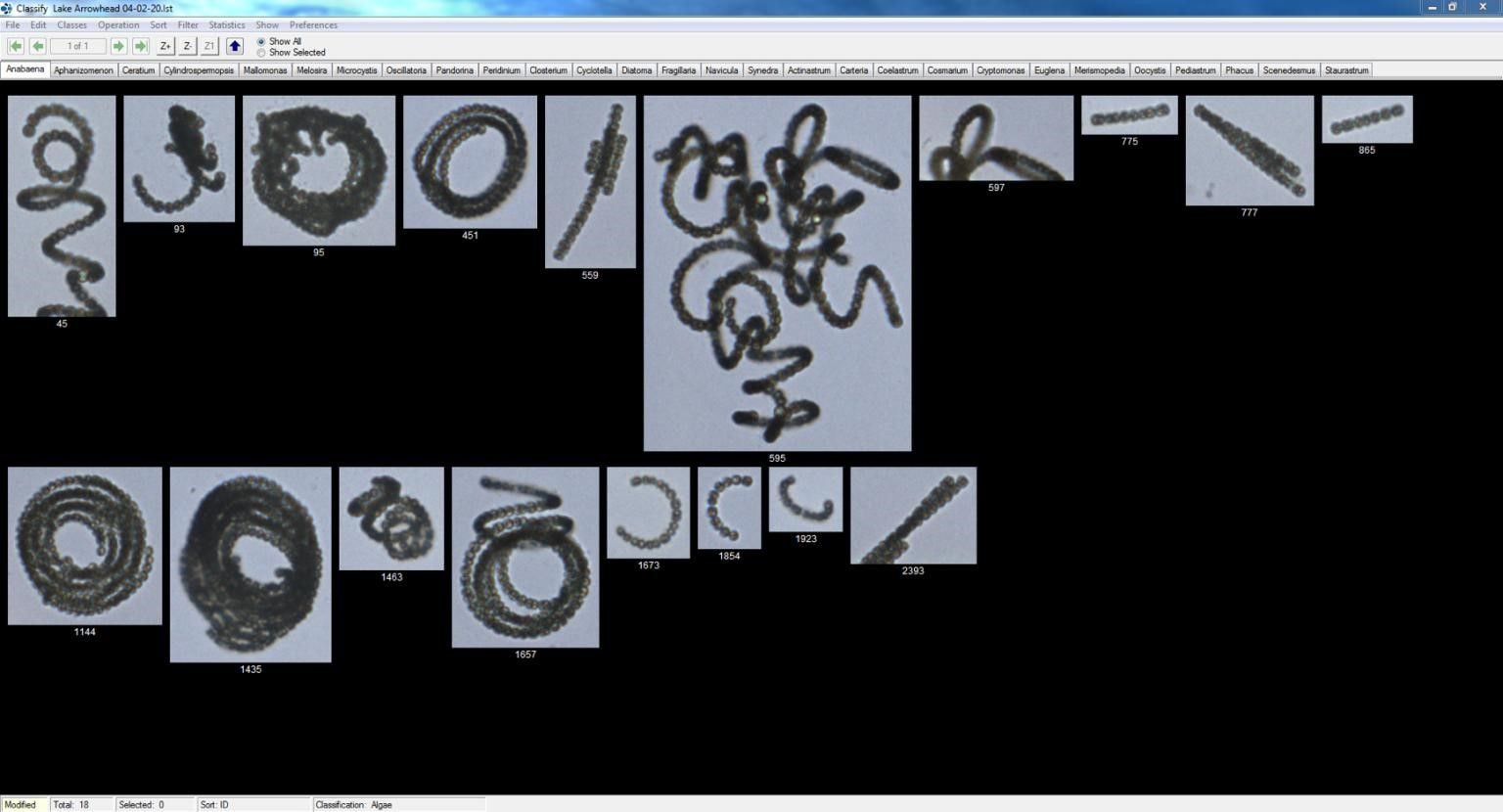
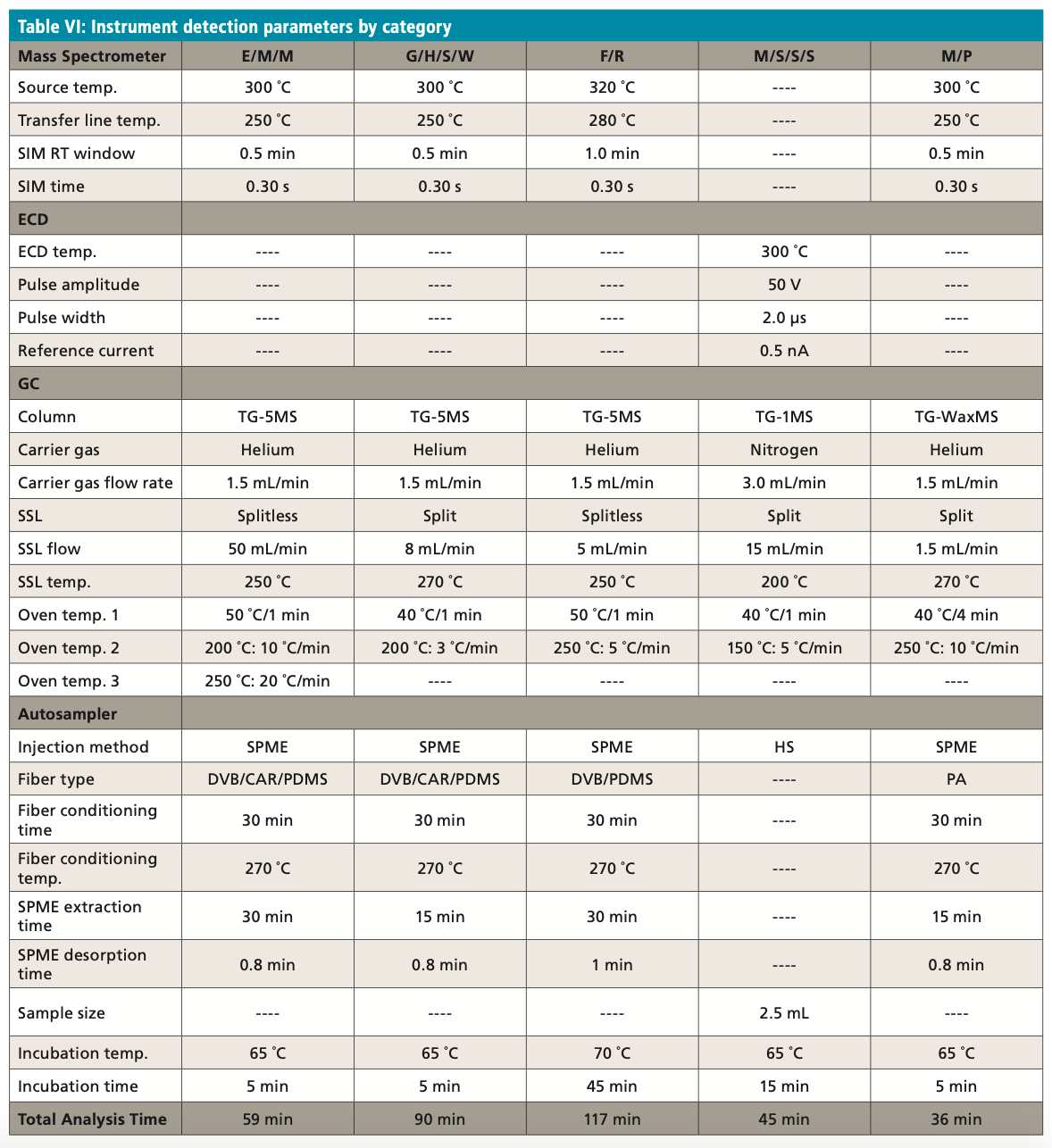
Over the last four years, the Cypress Environmental Laboratory, the municipal lab owned by the City of Wichita Falls, Texas, has detected 12 T&O events, totaling 17 months of active T&O mitigation. There were 11 events that were suspected to be a particular cyanobacteria taxon. The largest event in terms of duration and concentration occurred between February and May 2020, when an Anabaena bloom reached >1500 chains/mL and geosmin levels exceeded 15,000 ng/L (as seen in Figures 2 and 3). Each episode was detected and mitigated before the T&O-contaminated finished drinking water was released for public consumption. As a result, there have been no T&O customer complaints since the monitoring plan was activated in 2016. Instrument detection parameters for all categories can be found in Table VI. It is important to note that standards of the highest purity possible are used to ensure quantitative precision. Sigma Aldrich part numbers are listed with each compound in their respective category tables, but higher purity standards should be used when they become available.
Figure 2: Anabaena bloom reaching >1,500 chains/mL and >15,000 ng/L geosmin in a Lake Arrowhead sample from April 2020.

Figure 3: Anabaena bloom imaged using flow-imaging microscopy of a Lake Arrowhead sample from April 2020.
Conclusion
Although this protocol is designed to detect the primary compounds of interest identified on the T&O wheel, it is important to note that there are many others capable of causing events. The Cypress Environmental Laboratory continues to monitor source water samples for known and unknown T&O compounds and drives methodologies to ensure that the tap water remains T&O-free. Important research in this arena has been conducted in the last several decades, but overall, T&O work can still be considered in nascent stages, leaving ample room for new research. Aesthetic quality and perception of taste and odors remain some of the most problematic inhibitors of customer satisfaction in many cities, but that is no longer the case for the residents of Wichita Falls, Texas.
References
(1) E.N. Horsford and C.T. Jackson. Reports on Recent Impurities in the Water. Report Cochituate. City Document 9, 32-58. Water Board, City Council, Boston, Massachussetts (1855).
(2) American Public Health Association, Standard Methods of Water Analysis (American Public Health Association, Washington, D.C., 1st ed., 1905).
(3) R.B. Baird, A.E. Eaton, and E.W. Rice, Standard Methods for the Examination of Water and Wastewater (American Public Health Association, Washington, D.C., 23rd ed., 2017).
(4) T.F. Lin, S. Watson, A.M. Dietrich, and I.H. Suffet, Taste and Odour in Source and Drinking Water (IWA Publishing, London, United Kingdom, 2019). www.chromatographyonline.com.
(5) J. Force, Treatment Plant Operator 12, 10–15 (2016).
(6) M. Southard, H. Adams, and D. Nix, Taste & Odor Guidance Manual (City of Wichita Falls, Texas, 2016).
(7) American Water Works Association, M57 Algae: Source to Treatment (American Water Works Association, Denver, Colorado, 1st ed., 2010).
(8) F. Buerkens, S. Smith, G. Ford, and H. Adams, Opflow 46(10), 10–14 (2020).
(9) F. Buerkens, T. Kilgore, and H. Adams, Opflow 46(11), 16–19 (2020).
(10) P. Benskin, M. McKay, H. Adams, and F. Buerkens, Common Source Water Taxa: Algae and Cyanobacteria. A FlowCam Image Guide for Drinking Water Utilities (Yokogawa Fluid Imaging Technologies, Inc., Scarborough, Maine 2019). Retrieved from URL: https:// info.fluidimaging.com/hs-fs/hub/300163/ file-602141921.pdf.
(11) G.A. Burlingame, S.D.J. Booth, A. Bruchet, A.M. Dietrich, D.L. Gallagher, D. Khiari, I.H. Suffet, and S.B. Watson, Diagnosing Taste and Odor Problems Field Guide (American Water Works Association, Denver, Colorado, 1st ed., 2011).
(12) H. Adams, F. Buerkens, A. Cottrell, S. Reeder, and M. Southard. Opflow 44(12), 20, 21 (2018).
(13) W.D. Taylor, R.F. Losee, M. Torobin, G. Izaguirre, D. Sass, D. Khiari, and K. Atasi, Early Warning and Management of Surface Water Taste-and-Odor Events (American Water Works Association Research Foundation, Denver, Colorado, 1st ed., 2006).
(14) J.D. Wehr, R.G. Sheath, and J.P. Kociolek, Freshwater Algae of North America (Academic Press, San Diego, California, 2nd ed., 2015).
(15) J. Mallevaille and I.H. Suffet, Identification and Treatment of Tastes and Odors in Drinking Water (American Water Works Association Research Foundation, Denver, Colorado, 1st ed., 1987).
(16) I.H. Suffet, J. Mallevaille, and E. Kawczynski, Advances in Taste-and-Odor Treatment and Control (American Water Works Association Research Foundation, Denver, Colorado, 1st ed., 1995).
Hunter Adams, Mark Southard, Sam Reeder, Cali Barton, and Daniel Nix are with the City of Wichita Falls, Texas. Frances Buerkens is with Yokogawa Fluid Imaging Technologies Inc. in Scarborough, Maine. Direct correspondence to: hunter.adams@wichitafallstx.gov

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.










