Simultaneous Analysis of Ultrashort-Chain, Alternative, and Legacy PFAS in Potable and Non-Potable Waters
A hybrid HILIC–ion exchange column was used for the analysis of ultrashort-chain per- and polyfluoroalkyl substances (PFAS) compounds in environmental waters. This direct injection LC–MS method enables simultaneous measurement of ultrashort- chain, alternative, and legacy PFAS in potable and non-potable waters.
Ultrashort-chain, or C2 and C3, per- and polyfluoroalkyl substances (PFAS) are small and polar compounds, including trifluoroacetic acid, perfluoropropanoic acid, perfluoroethane sulfonate, and perfluoropropane sulfonate. Their ubiquitous presence in aquatic environments has become a major concern in regard to PFAS contamination. Given the high polarity of ultrashort-chain PFAS, it is impractical to include these compounds in regular PFAS monitoring with reversed-phase liquid chromatography. In this study, a hybrid hydrophilic interaction chromatography (HILIC)–ion exchange column was used for the analysis of ultrashort-chain PFAS. A full workflow of a direct injection method is presented for simultaneous measurement of ultrashort-chain, alternative, and legacy PFAS in potable and non-potable waters.
Ultrashort-chain, or C2 and C3, per- and polyfluoroalkyl substances (PFAS), are small and very polar compounds that contribute to at least 40% of total PFAS detected in many environmental waters (for example, rain, river, and ground waters) (1–3). Ultrashort-chain PFAS include trifluoroacetic acid (TFA), perfluoropropanoic acid, perfluoroethane sulfonate, and perfluoropropane sulfonate, with TFA being the most abundant and difficult to analyze chromatographically. Current practices for PFAS monitoring do not address the analysis of these newly trending ultrashort-chain compounds because of their insufficient retention on commonly used reversed-phase columns. On the other hand, analytical methods implementing anionic exchange chromatography often show too much retention (1) and poor chromatographic performance of ultrashort-chain PFAS. The challenge becomes even greater for simultaneous monitoring of ultrashort-chain, alternative, and legacy PFAS in a single method.
Using a unique hybrid hydrophilic interaction chromatography (HILIC)–ion exchange column, we developed a fast and simple liquid chromatography with tandem mass spectrometry (LC–MS/MS) method for comprehensive analysis of C2, C3, C4, C6, C8, and alternative PFAS. A direct injection method was evaluated for precision and accuracy analysis of fortified water samples including tap water, river water, groundwater, and wastewater effluent from publicly owned treatment works (POTW). This method provides convenient setup and high throughput analytical conditions for laboratories interested in adding ultrashort-chain compounds to an existing PFAS assay.
Materials and Methods
Chemicals and Solutions
Perfluoropropionic acid (PFPrA), perfluorobutanoic acid (PFBA), perfluorohexanoic acid (PFHxA), perfluorooctanoic acid (PFOA), perfluoropropanesulfonic acid (PFPrS), perfluorobutanesulfonic acid (PFBS), perfluorohexanesul-4,8-dioxa-3H-perfluorononano-fonic acid (PFHxS), perfluorooctaneate (ADONA), hexafluoropropyl-sulfonic acid (PFOS), ammoniumene oxide dimer acid (HFPO-DA), 9-chlorohexadecafluoro-3-oxanonane-1-sulfonate (9Cl-PF3ONS), and 11-chloroeicosafluoro-3-oxanonane- 1-sulfonate (11Cl-PF3OUdS) were obtained from Wellington Laboratories Inc. Trifluoroacetic acid (TFA) and perfluoropropionic acid (PFPrA) were obtained from Sigma-Aldrich. Perfluoroethane sulfonate (PFEtS) was obtained from Kanto Corporation. Formic acid and ammonium for- mate of analytical grade were obtained from Sigma-Aldrich. Acetonitrile for the mobile phase was obtained from Fisher Chemical. Methanol of HPLC grade for the mobile phase and Emsure methanol for sample preparation were obtained from EMD Millipore. Deionized water (generated by Thermo Scientific Barnstead E-Pure System) was used for the mobile phase and sample preparation.
Chromatographic Method
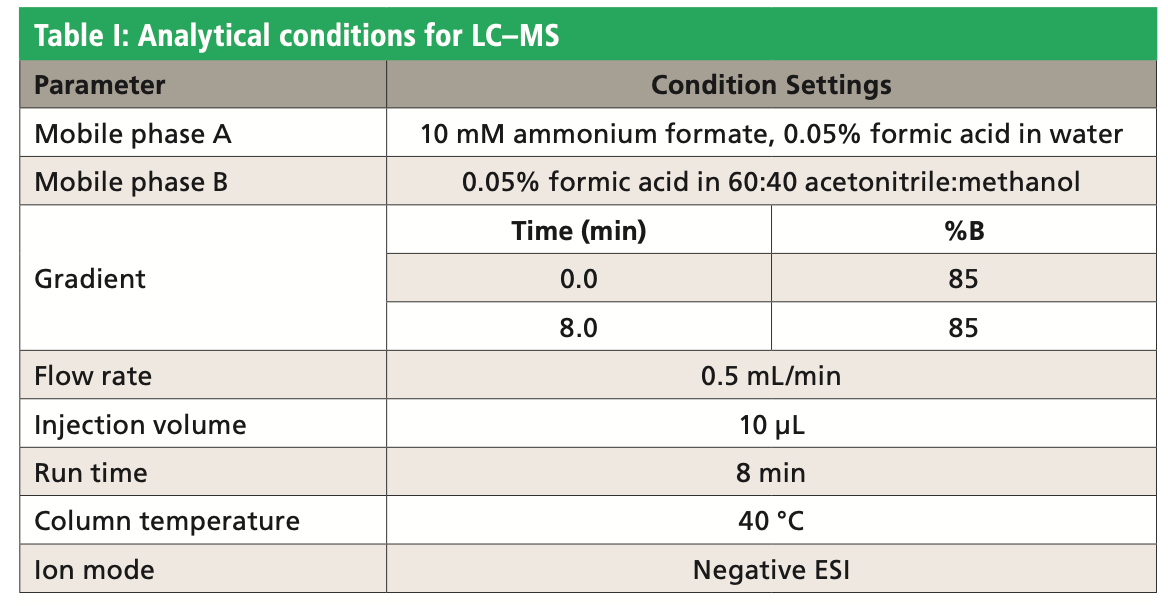
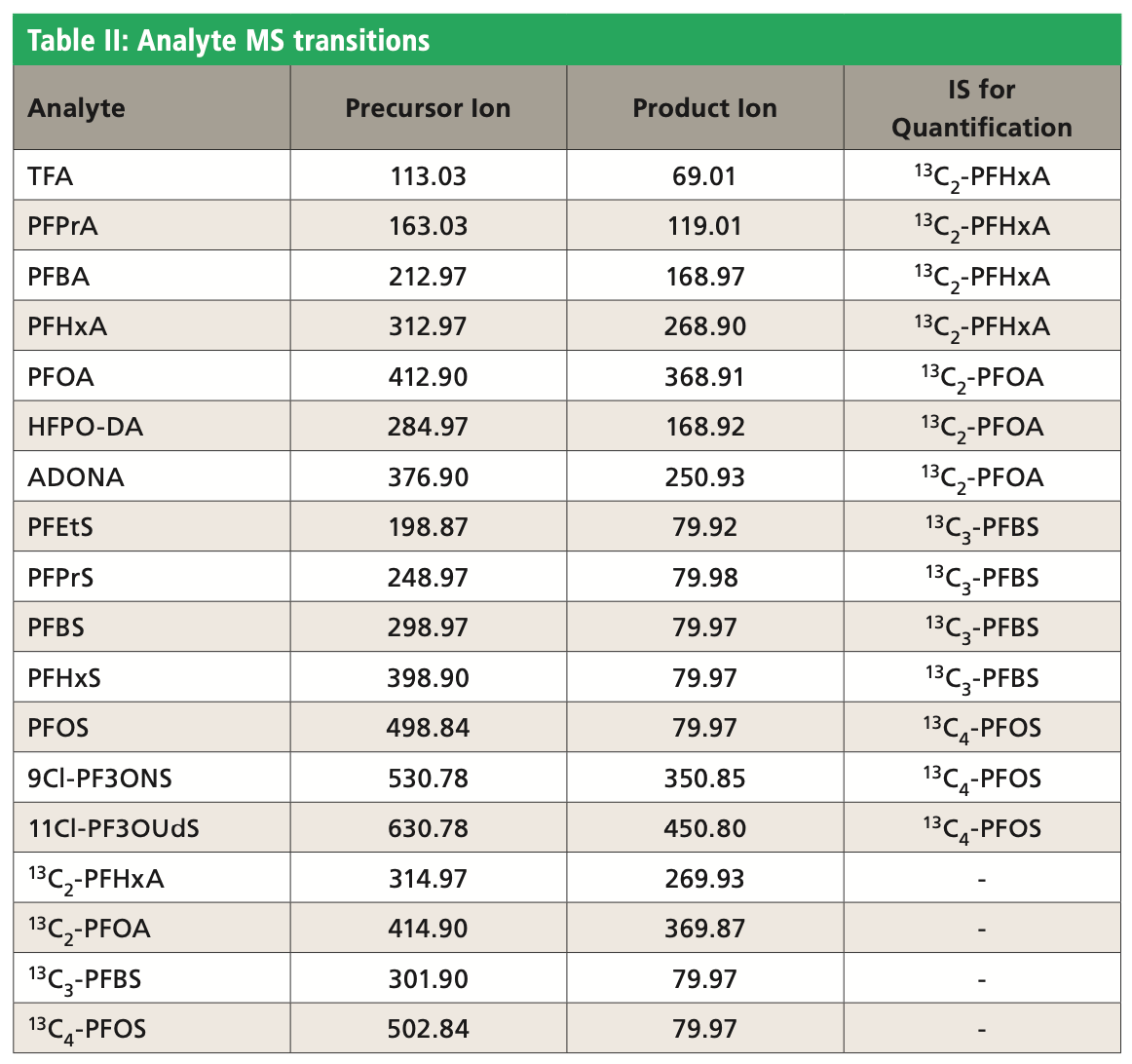
The chromatographic analysis was performed on the Raptor Polar X column (2.7-μm, 50 mm x 2.1 mm, Restek Corporation). Tables I and II show the chromatographic conditions and analyte MS transitions used in this work. A Waters Acquity UPLC instrument, coupled with a Xevo TQ-S triple-quadrupole mass spectrometer, was used for LC-MS analysis.
Sample Preparation
In a polypropylene vial, 250 μL of testing water sample was mixed with 250 μL of methanol and 5 μL of internal standard solution (10 ng/ mL of 13C2-PFHxA, 13C2-PFOA, 13C3-PFBS, 13C4-PFOS in methanol). The vial was capped with a polyethylene cap for injection analysis.
Calibration standards were prepared by fortifying 14 analytes in deionized water at a range of 10–800 ng/L. The calibration standard solutions were then diluted following the sample preparation procedure above.
A tap water sample from the Restek facility and three water samples (Chicago river water, groundwater, and POTW effluent water) supplied by the United States Environmental Protection Agency (U.S. EPA) were fortified at 40 and 160 ppt. Blank and fortified water samples were prepared as above for chromatographic analysis and quantified with the calibration standards. For TFA measurement in groundwater, the sample was diluted 5-fold with deionized water before fortification at 40 and 160 ppt due to its high TFA concentration.
Results and Discussion
Chromatographic Performance
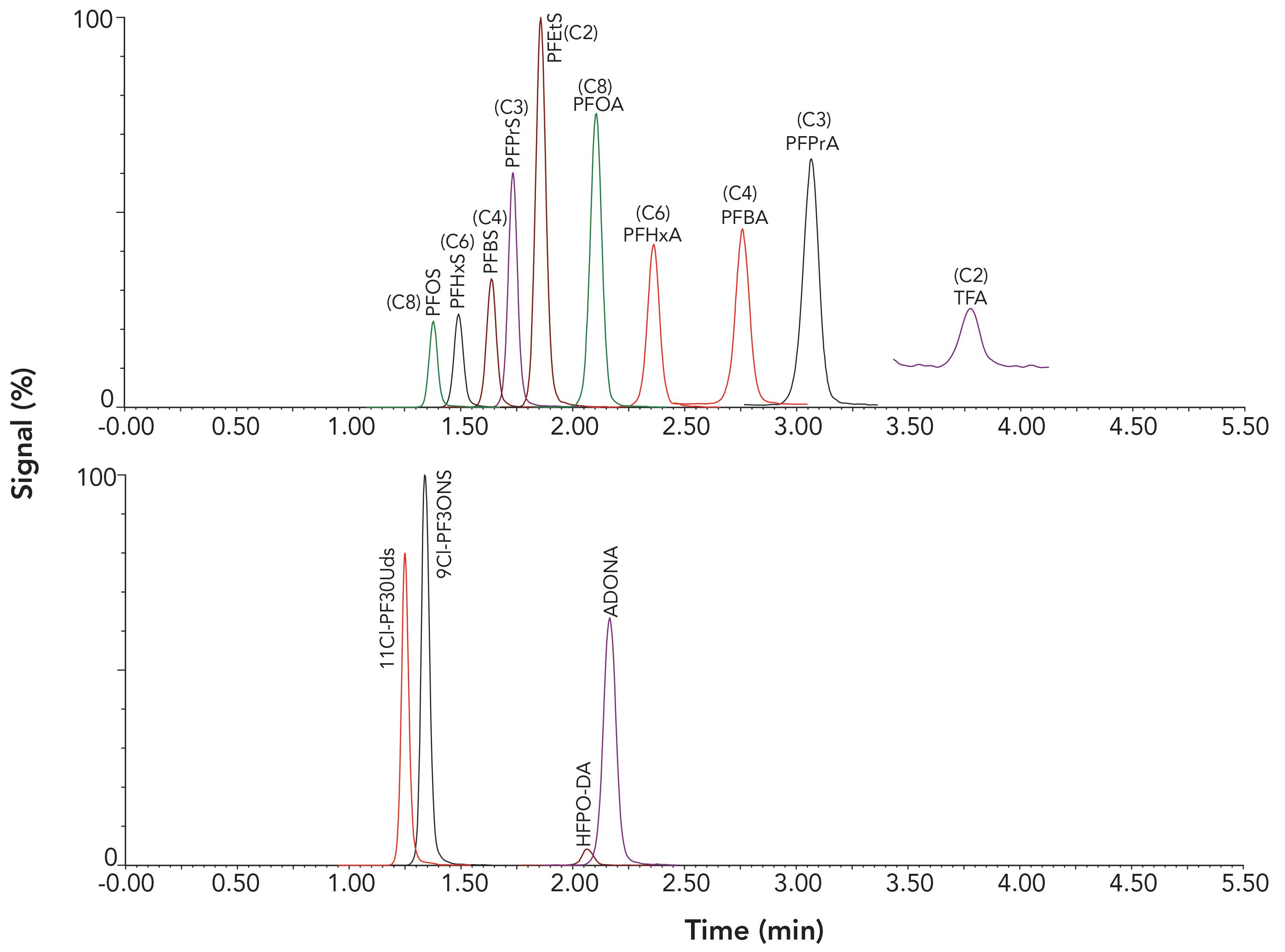
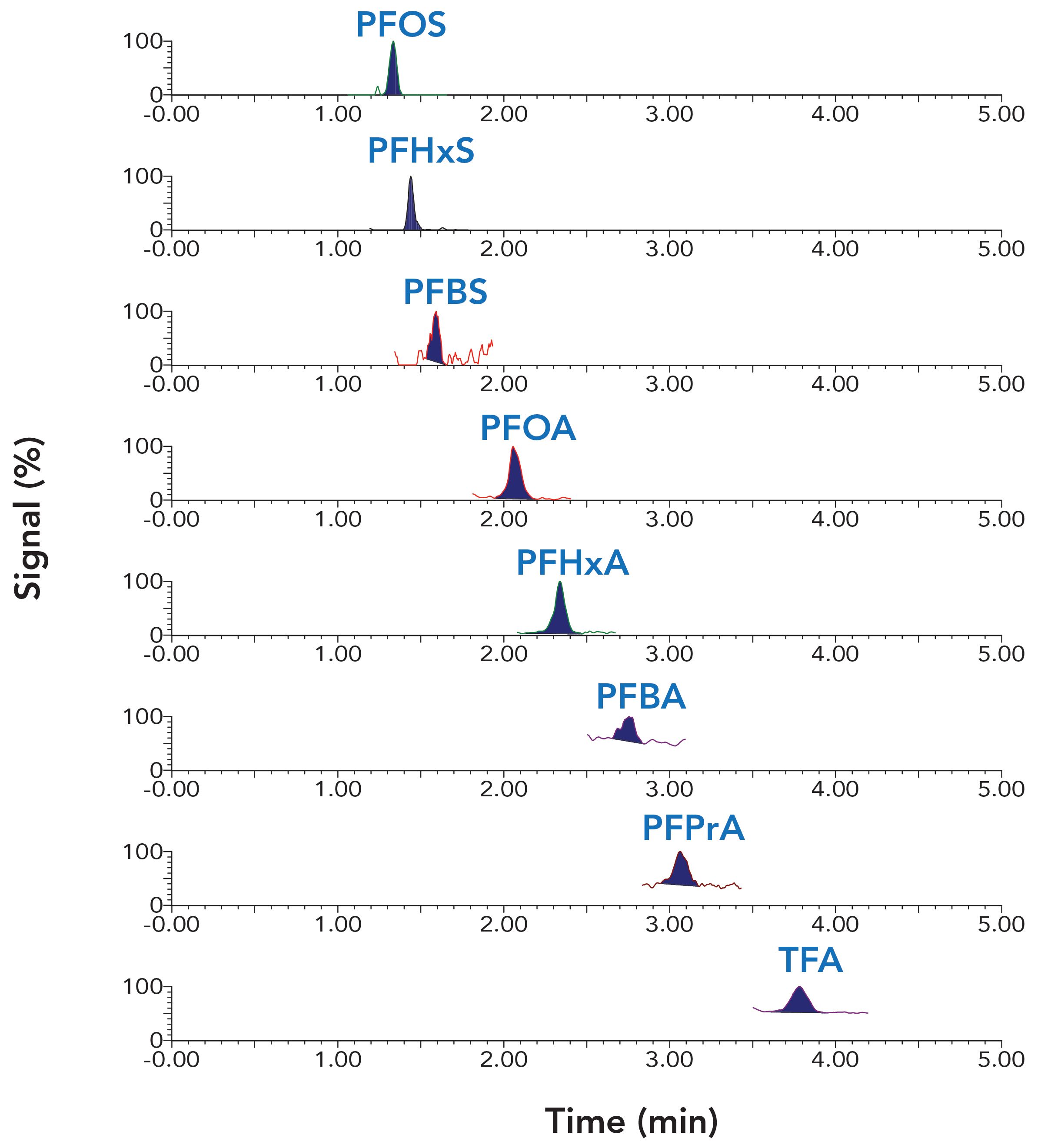
An isocratic elution with a higher percentage of organic mobile phase was established for a simple and fast chromatographic analysis. The addition of methanol in the organic mobile phase not only increases the detection sensitivity of PFAS, but also assists in the adjustment of proper retention and separation of these PFAS compounds. Figure 1 shows that all analytes were eluted within 4 min with balanced retention and good peak shapes. Matrix interference for the TFA signal was observed for water sample analysis with a shorter isocratic elution. Different elution times were tested, and it was determined that an 8 min run time was needed to avoid matrix interference for all analytes. This runtime may need to be modified based on specific instrumentation or the samples that are analyzed.
Figure 1: Chromatograms for the analysis of a 400 ppt standard solution in 50:50 water:methanol.
Linearity
The calibration range (before dilution) was 20–800 ppt for TFA and 10–800 ppt for all other analytes. Table II shows that four isotopic internal standards were evaluated for the best fitting standard curve of different analytes. All com- pounds showed acceptable linearity with r2 value >0.996 and deviations <20% using a quadratic regression (1/x weighted).
Accuracy and Precision
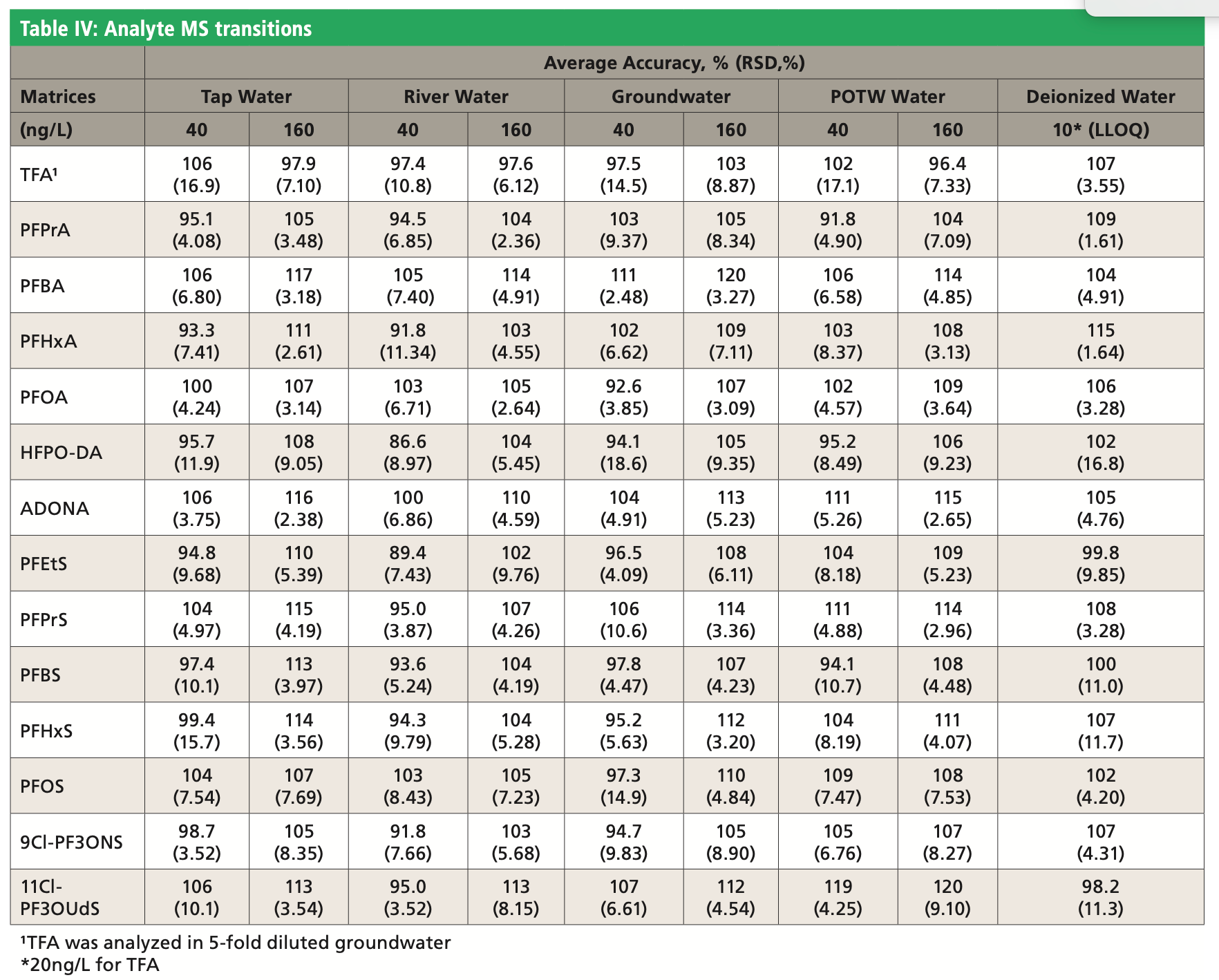
Table III shows that the blank water samples contained various levels of TFA, C3, C4, C6, and C8 PFAS with no detectable ADONA, HFPO-DA, 9Cl-PF3ONS, or 11Cl-PF3OUdS. An example chromatogram for the unspiked POTW sample is shown in Figure 2. For accuracy determination, the measured amounts in the fortified samples were adjusted to account for the concentration in unspiked samples for the recovery calculation. Water samples were fortified at low and high concentration in duplicate for each batch of analysis. A total of three batches of analyses were performed on different days. Table IV shows the accuracy and precision results calculated from the collection of all three batches of data. The method accuracy was demonstrated with recovery values within 30% of the nominal concentration for both fortified and lower level of quantification (LLOQ) levels in water samples. The relative standard deviation (RSD) was <20%, indicating acceptable method precision.
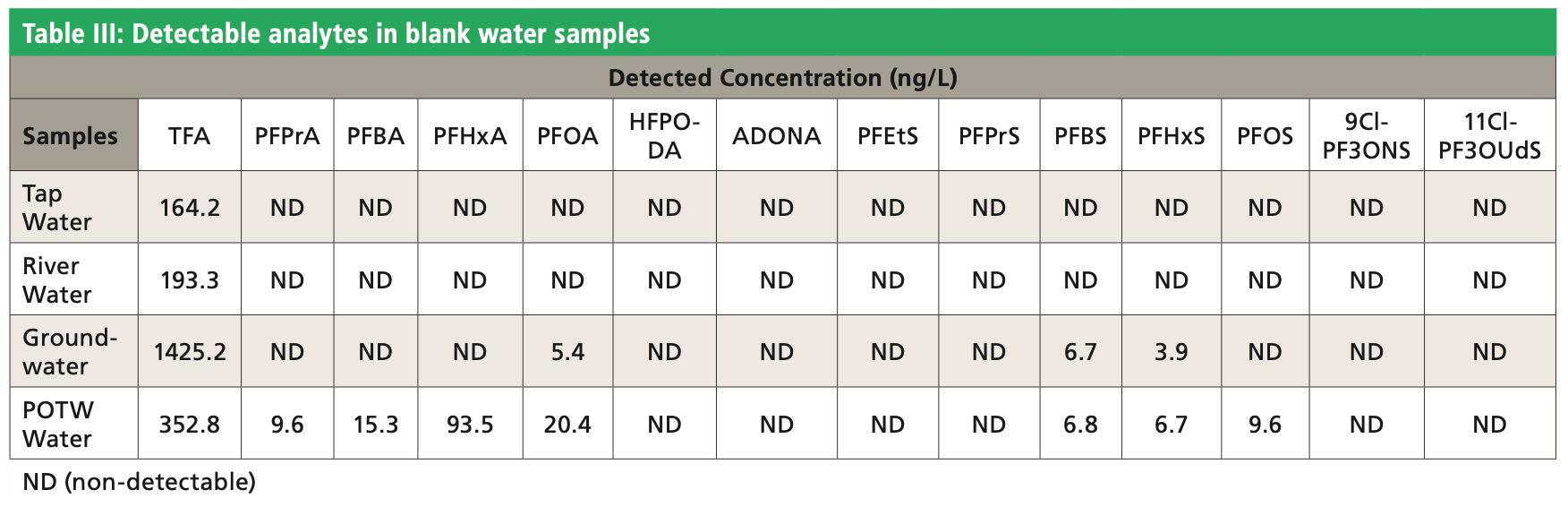
Figure 2: Detectable PFAS in blank POTW water.
Conclusions
A simple dilute-and-shoot method using a hybrid HILIC–ion exchange column was developed and validated for the simultaneous analysis of C2, C3, C4, C6, C8, and alternative PFAS in various water samples. The analytical method was demonstrated to be fast, rugged, and sensitive with acceptable accuracy and precision. This method is suitable for analytical laboratories wanting to expand their existing PFAS assays in drinking or non-potable water to include C2 and C3 compounds.
References
(1) S. Taniyasu, K. Kannan, L.W.Y. Yeung, K.Y. Kwok, P.K.S Lam, and N. Yamashita, Anal. Chim. Acta. 619, 221–230 (2008).
(2) K.Y. Kwok, S. Taniyasu, L.W.Y. Yeung, M.B. Murphy, P.K.S. Lam, Y. Horii, K. Kannan, G. Petrick, R.K. Sinha, and N. Yamashita, Environ. Sci. Technol. 44, 7043–7049 (2010).
(3) J. Janda, K. Nodler, H.J. Brauch, C. Zwiener, and F.T. Lange, Environ. Sci. Pollut. R. 26, 7326–7336 (2018).
Shun-Hsin Liang is a Senior Scientist at Restek Corporation. Justin Steimling and Xiaoning Lu are R&D Managers at Restek Corporation. Frances Carroll is a R&D Director at Restek Corporation. Connor Flannery is a Product Marketing Manager at Restek Corporation and Mike Chang is a Market Business Development Manager at Restek Corporation. Direct correspondence to: Shun-HsinLiang@restek.com.

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.










