Fast and Ultrafast HPLC on sub-2 μm Porous Particles — Where Do We Go From Here?
LCGC Europe
Higher productivity and faster analyses are two of the driving forces for continued improvement in high performance liquid chromatography (HPLC) column technology. Reduction in the average particle size of HPLC porous column packings below 2 ?m has resulted in sub-1.0 min separations in the gradient and isocratic modes. In this instalment of "Column Watch", Ron Majors traces the development of particle technology from the beginning of HPLC to the present, discusses why small particles are desirable, and probes some of the difficulties to be encountered, including extracolumn band broadening, pressure restrictions, and instrumental considerations. Finally, he shows a wide variety of fast- and ultrafast applications examples from commercial products in the sub-2 ?m range. Speculation on future directions in HPLC in particle technology concludes the column.
Since the beginning of modern high performance liquid chromatography (HPLC) in the late 1960s, users have required continually new and improved columns to tackle more difficult separation problems or to improve their overall productivity and sample throughput. Column researchers and manufacturers have responded to these needs with the development of more efficient and more reliable packing materials. One of the areas in which improvements have been made is in particle size reduction. Figure 1 shows a series of H versus ν curves that I developed in the early 1970s that showed the influence of the particle size of silica gel on column efficiency.1 Known by theoreticians for years, this data systematically showed that the use of smaller size particles resulted in more efficient columns. At that time, all we had to use were irregularly shaped particles; spherical microparticulate silicas had not yet been developed. To make an easier comparison, Figure 2 provides a plot of this data for H at 1.0 cm/s linear velocity versus average particle size of the silica packing. This log–log plot suggested that even smaller irregular particles would result in further performance improvements but at the time, smaller particles were not available in narrow particle size distributions.
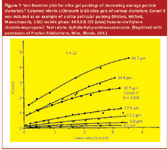
Figure 1
Figure 3 gives a rough chronological order of the introduction of commercial column packings since the beginning of HPLC. The first high-performance packings were the pellicular (porous-layer bead) ion-exchange packings developed by Horvath and coworkers.2 These particles were used for the separation of nucleotides and resulted in the first commercial high-performance packing Pellosil (Northgate Laboratories, Hartford, Connecticut, USA). Pellicular packings were rather large compared with today's particles — 40–50 μm. They had a thin porous coating that allowed rapid solute mass transfer into and out of the packing, resulting in improved chromatographic efficiency relative to the large porous particles that were generally used for liquid chromatography (LC) separations at the time. However, these pellicular packings had a big disadvantage — low surface area and, thus, very low sample capacity. For more details on this historical perspective, consult reference 3.
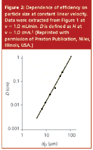
Figure 2
At the time, column researchers knew that small porous particles (less than 20 μm) would provide even better efficiency and maintain the high capacity of the earlier porous packings. Some earlier work by Piel4 in 1966 and Bidlingmeyer and Rogers5 in 1969 showed promise, but the particles that they used were commercially available Cabosil (Cabot, Billerica, Massachusetts, USA) fumed-silica packings that were sub-1.0 μm sizes, were fairly inert, were difficult to handle and required extremely high pressures to operate. In short, these particles were unsuitable for the current needs at the time. Small porous particles in the range of 10 μm were not available in narrow particle-size distributions and in commercial quantities and packing procedures for micrometer-size particles into usable columns were not available. This all ended when Merck (Darmstadt, Germany) produced 5–10 μm narrow cuts of their thin-layer chromatography (TLC) grade silica gel and made them commercially available, and high-pressure slurry techniques were developed to reproducibly pack them.6 The first microparticulate column, MicroPak Si-10 silica gel, was introduced in 1972 by Varian Associates (Walnut Creek, California, USA).
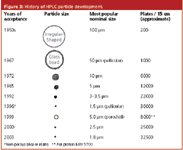
Figure 3
As a natural progression, as smaller particles were developed for HPLC, 5 μm became the standard particle diameter in the late 1980s. Later, high-performance 3 μm particles were reintroduced in the early 1990s after some initial performance problems of 3 μm commercial columns in the 1980s. Most recently, the sub-2 μm barrier was broken with the introduction of the Zorbax Rapid Resolution HT columns (Agilent Technologies, Palo Alto, California, USA) in 2003. As depicted in Table 1, at Pittcon 2005, column suppliers showed a number of high-performance columns packed with sub-2 μm particles.7
Interestingly, along the way of particle size reduction, the pellicular concept reappeared first with the development of the nonporous silicas8 and then with the poroshell silicas.9,10 The nonporous silicas (and also nonporous resins) were around 1.5 μm in average particle diameter and were best for the separation of macromolecules such as proteins. They provided rapid separations but had very low sample capacity and high-pressure drops so columns had to be rather short. The Poroshell silicas (Agilent Technologies) were of 5 μm diameter, so that pressures were reduced greatly and they had higher sample capacity than the nonporous silicas and resins. Poroshell columns provide fast separations of proteins, which diffuse very slowly and, thus, show poor mass transfer characteristics relative to small molecules. Both types of packings could be derivatized with various silane functionalities to perform reversed-phase, ion-exchange and affinity separations.

Table 1: Commercial Sub-2 μm HPLC Columns.
The purpose of this article is to focus on the smallest particle columns that appear to be directed to ultrafast separations using very short columns and to longer columns directed more for high-resolution separations of complex multicomponent samples.
Why Do Small Particles Give Better Chromatographic Performance?
The goal of LC is to separate as many molecules as possible in the shortest possible time using a high-efficiency column packed with small particles that interact with the molecules by various chemical forces. Ideally, one would like to inject a multicomponent sample as an extremely narrow band that would then be separated into discrete narrow bands of the individual molecules at the end of the column. Working against this process is band spreading, which occurs during the transit of the molecules down the column length. Starting with the actual injection itself, band spreading occurs in areas in which there is no packing to interact with the sample molecules. One source of band spreading occurs outside of the column itself and is referred to as extracolumn effects. This extracolumn band broadening occurs in the sample loop, ports of the injector body, connecting tubing and fittings, column frits, column end fittings, as well as the detector flow cell. Another source of band broadening associated with the mobile phase occurs within the chromatography column between particles and in the pores of the particles. Finally, mass transfer occurs as the solute molecules transfer from the stagnant mobile phase within the pore to the stationary phase and back out again.
Without getting too deeply into chromatographic theory, the separation efficiency H (or height equivalent to theoretical plate [HETP]) in micrometres as a function of mobile phase velocity is described by the van Deemter equation, shown simplistically in Equation 1.
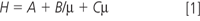
where A, B and C are constants and μ is the mobile phase linear velocity (proportional to flow-rate), measured in centimetres per second. The A term is a measure of the packing efficiency and is a function of packing efficiency and particle size. The B term is a function of longitudinal diffusion, or diffusion in the mobile phase, and the C term is a function of the mass transfer between the stationary and mobile phase as well as within the mobile phase. Within the C term, there is also a proportional dependency of the particle diameter squared.
Figure 4 shows a diagram of the additivity of the three terms in the van Deemter equation. Note that the B term is dominant at low flow velocities, while the C term is dominant at high flow velocities. The minimum of the van Deemter curve represents the ideal flow velocity where maximum column efficiency is obtained. It is a compromise between the B and C terms. Figure 4 is an idealized representation of the curves shown in Figure 1. The A and C van Deemter terms are influenced by the particle size. Smaller particles tend to reduce the value of H, which means that the column is more efficient — that is, it provides more theoretical plates per unit length. Small particles tend to allow solutes to transfer into and out of the particle more quickly because their diffusion path lengths are shorter. Thus, the solute is eluted as a narrow peak because it spends less time in the stationary and stagnant mobile phase where band broadening occurs.
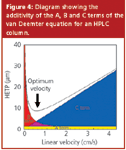
Figure 4
One advantage of using smaller particles is that the column can be shortened and the plate count remains the same or nearly so. A shorter column means a faster separation can be achieved because separation time is proportional to column length. A shorter column run at the same linear velocity as a longer column also uses less solvent.
Another fallout of the decrease in particle size is that the van Deemter curves tend to flatten out at higher linear velocities and the minimum shifts toward the right. Figure 5 shows a series of van Deemter curves for 5, 3.5 and 1.8 μm bonded spherical silica columns. One can easily see that the column packed with 1.8 μm particles gives a flatter curve at high linear velocity than the 5 μm column. Thus, one can run faster flow-rates (linear velocities) and peaks maintain their efficiency yet the separation time decreases proportional to the increase in flow-rate.
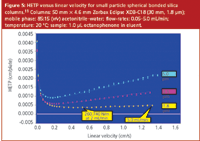
Figure 5
Are There Any Downsides to Reducing the Particle Size?
There are a number of experimental parameters one should be aware of when reducing the particle size. One is the column pressure. Equation 2 shows the dependence of column head pressure on a number of experimental parameters including the particle size. Note that the pressure is inversely proportional to the square of the particle size.
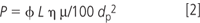
- P = pressure drop;
- φ = 500, flow resistance parameter;
- η = viscosity (mPa/s);
- μ = linear velocity (mm/s);
- L = column length (mm);
- dp = particle size (μm).
So when the particle size is halved, the pressure goes up by a factor of four. However, often for fast and ultrafast separations, the column length is also reduced so the pressure increase is not nearly as high as one would expect because pressure is proportional to column length. Of course, if longer lengths of columns, say 100 or 150 mm, are required to achieve higher plate counts, then higher pressure pumps might be required. Currently, there are commercial HPLC systems with upper pressure limits as high as 2 × 104 psig. It should be noted that the total pressure that the HPLC system experiences is the sum of the column backpressure and the instrument backpressure. The latter results when small internal diameter capillaries are used in the flow paths to reduce extra column effects and the gradient delay volume. As the flow-rate increases, the back pressure due to these capillaries increases proportionally.
Another experimental parameter that bears watching when reducing the column length and especially when reducing the internal diameter is the extracolumn band broadening. Some of the modern ultrafast LC columns are only 15 mm long with an internal diameter of 2.1 mm. Such a column has a total void volume of around 33 μL. Many conventional HPLC instruments were developed for typical 150 and 250 mm × 4.6 mm analytical columns, where the total column void volumes are 1.6 and 2.6 mL, respectively. Peaks on the 15 mm × 2.1 mm column when packed with 1.8 μm particles are often only a few microlitres wide (for low-k peaks), which implies that extracolumn band broadening must be minimized if the true advantages of these small columns are to be realized. Some modern liquid chromatographs have been designed or modified to minimize the extra column effects. Upgrade kits are available to modify some older HPLC instruments to work satisfactorily with sub-2 μm columns. Extracolumn band broadening and similar effects are more thoroughly discussed in reference 11.
Some other experimental parameters that must be taken into account when running fast and ultrafast separations are as follows:
- Detector time constant (peaks can be only a second or two wide for short, narrow-bore columns run at high flow-rates; and a time constant that is too slow will make the peaks artificially broad because the detector cannot keep up with the rapidly changing signal)
- Data sampling (acquisition) rate (data system needs enough data points, usually 10–20, across a peak to define the peak to integrate area, determine retention time and so forth)
- Autosampler cycle time (for separations less than a minute or two, throughput can be slowed down by a slow autosampler)
- Gradient delay volume (if the volume from where the gradient forms to the column head is too large, fast separations will be compromised because gradient has not reached the column in time).
So What is Fast and Ultrafast LC?
The terms "fast" and "ultrafast" are relative terms. If your separation was already 25 min on your present column and was reduced to 7 min, you would consider the latter time fast. If you were faced with hundreds of samples each requiring 7 min of separation time including gradient regeneration, then performing the same tasks in 2 min would be a big improvement and perhaps enable you to complete your task in a day or so instead of a week.
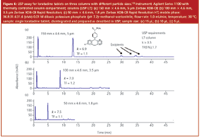
Figure 6
Basically, fast or ultrafast LC relies on the use of small particles packed into short columns run at high flow-rates. Often, one can accomplish the same separation that can be accomplished on a longer column with a larger particle size but in a fraction of the time. An example of a United States Pharmacopeia (USP) method that has been "downsized" is shown in Figure 6, where an antihistamine was chromatographed on three different columns packed with 5, 3.5 and 1.8 μm reversed-phase C18 bonded material with the same bonded stationary phase. Note that 150 mm × 4.6 mm columns packed with 5 μm particles are still the standard in most HPLC laboratories. This isocratic USP method required a total of 38 min [Figure 6(a)] on the 150 mm column. However, one can see that the same separation was achieved on a column [Figure 6(c)] that was only 50 mm in length but in a third of the time — in less than 13 min. Even switching to a 100 mm column reduced the total time to just over 23 min [Figure 6(b)]. Note that the excipients were separated on all three columns so nothing is lost in the "downsizing" experiments. The gain is separation speed.
Ultrafast separations generally refer to separations achieved in a minute or two for relatively simple samples. Figure 7 shows the 24 s separation of nine alkylphenones on a short (30 mm) column packed with 1.8 μm packing run at 5 mL/min. A ballistic gradient was used for the rapid elution of these nonpolar analytes. Because the average peak width was only 0.3 s, a diode-array detector with a fast sampling rate (80 Hz) was required. Otherwise, the fast peaks would have been distorted because the time constant would have been too slow to adequately detect them.
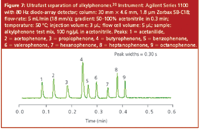
Figure 7
Where Can I Obtain Sub-2 μm Columns to Experiment With?
At Pittcon 2005, a number of companies displayed their newest small particle columns. They are listed in Table 1. I will elaborate on each of their offerings:
Agilent Technologies: The company offers a variety of their regular phases in the short, fast configurations, all packed with 1.8 μm versions of their 3.5 and 5.0 μm particles. Phases include Zorbax StableBond C8 and C18 for low-pH operation, Zorbax XDB-C8 and C18 for general-purpose separations, Zorbax Extend C18 for high-pH applications, and Zorbax-SB CN, which provides a different reversed-phase polarity. Both cartridges and standard compression fitting hardware is available in 2.1, 3.0 and 4.6 mm internal diameters. Column lengths of 15–100 mm are available. Relative to other sub-2 μm columns, pressure drops are reduced by purposely widening the particle size distribution without influencing column efficiency.12
Alltech Associates (now part of Grace Davison group), Deerfield, Illinois, USA: The company offers a 1.5 μm version of their regular columns that are packed with 3.0 and 5.0 μm particles. The company's Platinum HPLC columns have controlled surfaces that offer dual mode separations and extend the range of polar selectivity. The C8, C18 and extended polar selectivity (EPS) phases are available in 33 and 53 mm × 7 mm columns in the Rocket hardware format. These are silica-based columns with a 100 Å pore size and, thus, most appropriate for small-molecule separations. Available in the same hardware are two speciality columns: Alltima HP HILIC and ProSphere HP ZAP! C18. The former nonbonded, high-purity bare silica column is recommended for the hydrophilic interaction chromatography (HILIC) separations of highly polar compounds that are poorly retained or unretained on conventional reversed-phase columns. These columns are used with mobile phases consisting of mostly organic solvents with only small amounts of water in the mobile phase and are useful in LC–mass spectrometry (MS) for higher sensitivity with volatile mobile phases. For MS, a smaller 2.1 mm i.d. column is available. Columns of 10, 20 and 33 mm lengths are provided. The ProSphere column has a 500 Å pore size, which makes it ideal for the high-speed reversed-phase separation of proteins. Figure 8 gives an application of this column for the rapid (2.5 min) separation of proteins using a fast water–acetonitrile (each containing 0.1% trifluoroacetic acid) gradient.

Figure 8
Bischoff Chromatography, Leonberg, Germany: The firm offers four columns, three that are totally porous (1.8 μm) and one that is a nonporous silica phase (1.5 μm). The totally porous packings are based upon a 300 m2/g silica. ProntoPEARL sub-2 TPP-C8ace EPS (8% carbon loading) and C18 EPS (16% carbon) are smaller particle versions of their regular offerings. Column dimensions: 30–50 mm × 2.0 and 4.6 mm. The third phase on totally porous silica is the ProntoPEARL sub-2 TPP APS, which is a reversed phase with a polar-embedded functionality (3.5% carbon content). This packing gives higher retention for acidic compounds compared with C8 and C18 bonded phases. This 1.8 μm column was used to determine the polyphenol content of German red wine (Figure 9). Polyphenols are thought to be very healthy because they are antioxidants and their daily consumption might reduce the risk of coronary heart disease. Relative to the matrix components, the polyphenols were well retained; thus, no extensive sample preparation was required (only filtration through a 0.2 μm filter), and the wine was directly injected without dilution. This phase also separated the cis- and trans-resveratrol using isocratic elution conditions. At 2 mL/min, the column pressure was 28.0 MPa (4000 psi), well within the capability of most HPLC systems. The entire separation required less than 4 min.
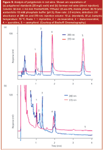
Figure 9
Thermo Corporation, Waltham, Massachusetts, USA: Hypersil Gold columns are based upon high-purity silica and are especially recommended for improving peak shape for basic compounds that tail on many reversed-phase columns. The introduction of the 1.9 μm columns at Pittcon 2005 complemented the line of 3, 5 and 8 μm Hypersil Gold reversed-phase columns already on the market. Three lengths (20, 30 and 50 mm) were introduced all with a 2.1 mm internal diameter. To illustrate the performance of these new columns, Figure 10 shows a rapid gradient chromatogram of seven beta-blocker pharmaceuticals. Beta blockers are a class of drugs that block beta-adrenergic substances and, thus, relieve stress on the heart and are used for treatment of cardiac arrhythmias, angina pectoris and hypertension. The separation was performed using a 20 mm Hypersil GOLD 1.9 μm column with a simple formic acid–water–acetonitrile mobile phase system. Using a ballistic gradient, the entire separation required only 1 min.

Figure 10
Waters Corporation, Milford, Massachusetts, USA: ACQUITY columns are the company's second-generation hybrids designed to work at higher pressures with the ACQUITY UPLC system. The silica–organic hybrid chemistry is based upon bridged ethylene groups within a silica gel particle structure giving the particle added mechanical strength and pH stability from pH 1 to pH 12, depending on the chemistry. The packings are endcapped. Several phase chemistries are available: C8, C18, embedded polar and C6 phenyl. Ligand densities range from 3.0 to 3.3 μmol/m2 . The pore diameter is 135 Å The C18 and C6 Phenyl phases can be used at temperatures as high as 80 °C. To illustrate the separation capability of the company's 1.7 μm column, the separation of seven substituted coumarins is depicted in Figure 11. Coumarin and its derivatives are principal oral anticoagulants. A rapid (ballistic) linear gradient gave a separation requiring less than 80 s on a 30 mm × 2.1 mm ACQUITY reversed-phase column.
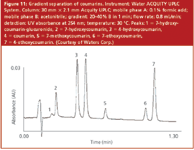
Figure 11
Future Directions in Small-Particle Technology
With separation speeds of relatively simple samples already in the subminute range using sub-2 μm columns, further reductions in porous particle size could result in even shorter columns with separations requiring only a few seconds. The question always arises as to what applications will need such rapid separations because such speeds will definitely tax current instrumentation. The rapid feedback required in on-line process analytical technologies could be one area that might create such a need. The screening of million-compound libraries in combinatorial chemistry and drug discovery could be another.
Alternatively, if higher plate counts are required, then longer columns with these smaller particles will be required. Already research groups of Jorgenson,14 Lee15 and Colon16 have demonstrated separations at pressures as high as 7000 bar using nonporous particles with diameters as small as 1 μm packed into nanobore columns (50 μm i.d.) to keep heat generation minimized. Such columns are capable of generating several hundred theoretical plates in a matter of minutes. However, a recent paper by Guiochon and Martin12 cautions users about working with such high pressures because of the effect of pressure on common experimental parameters and anticipated difficulties in method development and reproducibility. The jury is still out on what pressures will be required to obtain satisfactory performance. Of course, safety in the routine use of high pressures in the chromatography laboratory is always a consideration.17
Nevertheless, if the need for further reductions in particle size below the currently available 1.5–1.9 μm particles is required, no doubt manufacturers will respond to provide such columns. Some of the issues surrounding the optimum use of these micrometer-sized particles are the implementation of new column and instrument hardware designs; the development of efficient packing techniques; considerations of particle and packed-column stability; the ability to make stable wide pore packings for the separation of biomolecules. Silica gel-based packings become more friable as the pore size increases. Perhaps some of the techniques used to construct silica-organic hybrids, for the synthesis of highly crosslinked polymers, or the use of carbon or other inorganic-based packings could alleviate concerns in packing stability.
"Column Watch" editor Ronald E. Majors is Business Development Manager, Consumables and Accessories Business Unit, Agilent Technologies, Wilmington, Delaware, USA and is a member of the Editorial Advisory Board of LCGC Europe.
Direct correspondence about this column to LCGC Europe, Advanstar House, Sealand Road, Park West, Chester, CH1 4RN, UK, e-mail: dhills@advanstar.com
References
1. R.E. Majors, J. Chromatogr. Sci., 11, 88–95 (1973)
2. Cs.G. Horvath, B.A. Preiss and S.R. Lipsky, Anal. Chem., 39, 1422–1428 (1967).
3. R.E. Majors, LCGC, 12(7), 508–518 (1994).
4. E.V. Piel, Anal. Chem., 38, 670–672 (1966).
5. B.A. Bidlingmeyer and L.B. Rogers, Sep. Sci., 4, 439–446 (1969).
6. R.E. Majors, Anal.Chem., 44, 1722–1726 (1972).
7. R.E. Majors, LCGC, 23(3), 248–265 (2005).
8. T. J. Barder et al., LCGC, 15, 918–926 (1997).
9. J.J. Kirkland, F.A. Truszkowski and C.H. Dilks Jr., J. Chromatogr. A, 890, 3–13 (2000).
10. J.J. Kirkland, F.A. Truszkowski and R.D. Ricker, J. Chromatogr., 965, 25–34 (2002).
11. R. E. Majors, LCGC, 21(12), 1124–1133 (2003).
12. W.E. Barber, A. Broske and T. Langlois, "Influence of particle size distribution on HPLC column HETP and operating pressure: Design implications for very small particle packings, which generate high pressures," HPLC 2005, Stockholm, Sweden, June, 2005, Paper #P5:04.
13. A.D. Jerkovich, J. Scott Mellors and J.W. Jorgensen, LCGC, 21(7), 600–610 (2003).
14. N. Wu, J.A. Lippert and M.L. Lee, J. Chromatogr., 911, 1–12 (2001).
15. L.A. Colón et al., Analyst, 129, 503–504 (2004).
16. M. Martin and G. Guiochon, J. Chromatogr.A, 1090, 16–38 (2005).
17. Y. Xiang, D.R. Maynes and M.L. Lee, J. Chromatogr. A, 991, 189–196 (2003).
18. J.W. Henderson, Agilent Technologies, Palo Alto, California, Application Note Publication #5989-2908EN, 2005.
19. W.E. Barber et al., Pittcon 2004, Paper 13700–200, 10 March, 2004 (Chicago, Illinois, USA).
20. S. Schuette, A. Gratzfeld-Huesgen and A. Fandino, HPLC 2005, Stockholm, Sweden, Paper # TuL4:1, June 2005
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.