A Chromatographic Screening Method to Identify Agents that Reverse Intrinsic Drug Resistance in Colon Cancer Cells
LCGC Europe
Conjugation catalysed by the UDP-glucuronosyltransferase (UGT) superfamily of drug-metabolizing enzymes is an important mechanism of anticancer drug resistance in colon cancer cells. The mechanism manifests itself by a reduction in the intracellular concentrations of the parent drug through increased export of the glucuronide metabolites to the extracellular compartment. Modulation by an inhibitor of UGT inhibits the formation of metabolites and restores intracellular concentrations of the drug. This article describes a screening method using HT29 human colon cancer cells and based on HPLC methodology that allows the identification of effective modulators of the glucuronidation mechanism of drug resistance. A rapid solid-phase sample preparation technique using C2-bonded 40 ?m silica particles was developed for the extraction of cell lysates and culture media without degradation of unstable parent compounds and their glucuronides or artefactual in situ formation of metabolites.
Introduction
Although there are presently more than 50 active chemotherapeutic agents available to treat cancer, the disappointingly low rate of long-term survival in certain disease types, such as lung, gastrointestinal and kidney cancer, is largely because of the phenomenon of drug resistance.1 Anticancer drug resistance can be classified into two basic types: intrinsic or de novo drug resistance and acquired drug resistance. The phenomenon is often referred to as multidrug resistance (MDR).2

Conjugation catalysed by the UDP-glucuronosyltransferase (UGT, EC 2.4.1.17) superfamily of drug-metabolizing enzymes has recently been identified as a novel mechanism of drug clearance and intrinsic drug resistance in colon cancer.3–5 The mechanism operates with both SN–38 (active metabolite of the clinically used chemotherapeutic agent Irinotecan) and the novel anticancer compound NU/ICRF 505 (for structures see Figure 1) and manifests by a reduction in intracellular concentrations of the parent drug and increased export of the glucuronide metabolites to the extracellular compartment.
SN–38 has long been shown to be only active in a closed (ring E) lactone configuration but can undergo spontaneous ring opening to an inactive water soluble hydroxy acid at neutral to basic pH.6,7 Chromatographic methods for SN–38 have either not attempted to resolve the two different forms by sample acidification followed by protein precipitation with organic solvents or have required the addition of ion pairing agents and/or high ionic strength buffers to the mobile phase making these methods incompatible with mass spectrometry, again often without significant sample clean-up.8–14 Solid-phase extraction has rarely been applied in SN–38 analysis and here variable results have been reported.15 NU/ICRF 505 is also biologically unstable undergoing esterase catalysis to a more water soluble free amino acid hydrolysis product termed NU/ICRF 505/M.16 Often extreme conditions are required to resolve NU/ICRF 505 from NU/ICRF 505/M, such as a mobile phase consisting of 0.25 M ammonium acetate adjusted to pH 3 with 25% trifluoroacetic acid.17
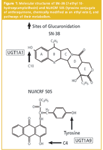
Figure 1
In this article we present chromatographic methods capable of separating these two parent compounds from their respective hydrolysis products and glucuronide metabolite(s) in a single run. In addition, a rapid sample preparation technique based on solid-phase extraction is presented that preserves the stability of the parent compound but also allows the glucuronide metabolites to be determined in cell lysates and tissue culture media. Using this methodology thus facilitates the in vitro screening of modulators of the glucuronidation resistance mechanism in colon cancer cells.
Experimental
Materials: The two glucuronide metabolites of NU/ICRF 505 (the ring C4-O-glucuronide and tyrosine glucuronide) and the single glucuronide metabolite of SN-38 (the C10-O-glucuronide) were biosynthesized by incubation with HT29 cells.3 These metabolites are shown in Figure 1.
NU/ICRF 505/M, the product of hydrolysis of the C-terminal ethyl ester linkage yielding the free amino acid was as described in a previous paper.16 SN–38 lactone was from Rhône-Poulenc Rorer (Vitry, France) and SN–38 hydroxy acid was prepared by alkaline hydrolysis of the parent compound.18 All common UGT substrates (aglycones) used were of the highest grade available commercially and were either from Aldrich or Sigma Chemical Co. (Poole, UK).
Cell lines and tissue culture: Transport of NU/ICRF 505 and SN–38 in HT29 (proficient in glucuronidation) and HCT116 (deficient in glucuronidation) human colon cancer cells were conducted in the presence of the following UGT aglycones: propofol, ibuprofen, methyl 4-hydroxybenzoate and R- and S-citronellol. These studies were performed in 12.5 cm2 tissue culture flasks with cells plated at a density of 106 cells in 4 mL of RPMI medium.19 Drugs were added at a final concentration of 10 μM for NU/ICRF 505 and 1 μM for SN–38 in the presence of 0, 50, 100 and 200 μM of the different UGT aglycones. At different time points, cells and tissue culture media samples were collected for quantitative determination of the two anticancer drugs, their glucuronide metabolites and hydrolysis products, by reversed-phase HPLC with solid-phase extraction.
High performance liquid chromatography: The HPLC used throughout consisted of an Alliance 2690 separations module, a 996 photodiode array detector set to record signals at 254 or 545 nm for NU/ICRF 505 and a 474 scanning fluorescence detector (SFD) set at 380 nm for excitation and 430 nm for emission to detect SN–38, all from Waters Ltd (Watford, UK). Full system control, data collection, analysis and reporting were performed using Millennium Software (revision 2.21, Waters). The stationary phase was Symmetry Shield (RP8) 5 μm particle size packed in a 150 × 3 mm internal diameter stainless steel analytical column and 20 × 3.8 mm diameter stainless steel pre-column (Waters). The mobile phase comprised 10 mM ammonium acetate as buffer A and methanol as solvent B.
Gradient elution was employed according to the following linear programme for NU/ICRF 505: time zero, 40% solvent B; 15 min, 100% solvent B; 17 min, 40% solvent B. Gradient elution was also employed for SN–38 according to the following linear programme: time zero, 20% solvent B; 15 min, 80% solvent B; 17 min, 20% solvent B. Flow-rate was 0.35 mL/min; total run time was 23 min, while columns were maintained at a temperature of 40 °C and the autosampler at a temperature of 20 °C. Mobile phase components were filtered prior to use and vacuum degassed in situ during chromatography. Injection volume was 50 μL.
Sample preparation by solid-phase extraction: Samples from the drug transport studies in colon cancer cell lines either containing 1 mL of tissue culture medium or 3 mL for cell sonicates (see later) were processed by SPE using C2 bonded 40 μm silica particles packed in 1 mL capacity mini-columns containing 100 mg of sorbent operating under negative pressure (Varian Associates supplied by Phenomenex, Macclesfield, UK). The mini-columns were activated with 1 mL of methanol and conditioned with 1 mL of 50 mM Tris–HCl (pH 7.4). Samples were then loaded onto the columns, which were then washed with 2 mL of 50 mM Tris-HCl (pH 7.4). The compounds of interest were finally eluted in 400 μL of methanol:1M ammonium acetate (90:10, v:v).18,20
Sample preparation of tissue culture cells: At specified time points, 1 mL of medium was removed from the tissue culture flask for drug analysis, cell mono-layers were washed twice with 4 mL of ice cold phosphate buffered saline (PBS), lysed by the addition of 2 mL of ice cold water and then mechanically detached from the plastic by scraping with the addition of a further 1 mL of ice cold water. Efficacy of the cell lysis protocol was confirmed using a particle size analyser (Model ZM, Coulter Electronics, Luton, UK). Finally, the lysed cell suspensions (3 mL) were subjected to further disruption by two brief (15 s) periods of sonication while being maintained at 0 °C in ice. Both media and cell samples were processed as quickly as possible to prevent in situ conversion of the SN–38 lactone to the hydroxy acid.
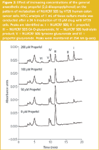
Figure 2
Results and Discussion
Modulation of NU/ICRF 505 glucuronidation in HT29 cells by propofol: The UGT superfamily currently consists of 18 different isozymes; nine are encoded by the UGT1A locus and nine are encoded by UGT2 genes.21,22 The HT29 cell line has been demonstrated by RT–PCR techniques by us to express every member of the UGT1A locus as well as key UGT2 genes, such as 2B7 and 2B15 and thus is an ideal cell line to study modulation of glucuronidation. HT29 cells exhibit a 5-fold level of drug resistance to NU/ICRF 505 and a 2-fold level of drug resistance to SN-38.4 The effect of increasing concentrations of the selective UGT1A9 substrate propofol (2, 6-diisopropylphenol) on the glucuronidation of NU/ICRF 505 by HT29 cells is shown chromatographically in Figure 2. In this experiment the accumulation of metabolites was determined in the tissue culture medium after a 24 h incubation with NU/ICRF 505. The Symmetry Shield column was able to resolve NU/ICRF 505 (peak 1) from its hydrolysis product NU/ICRF 505/M (peak 4), its C4-O-glucuronide (peak 3) and tyrosine glucuronide (peak 5), propofol (peak 2) and the glucuronide of propofol (peak 6); all with extremely good peak shape and high efficiency, without the need to add high ionic strength buffers to the mobile phase or resort to extremes of pH. In the absence of propofol, NU/ICRF 505 was extensively metabolised by HT29 cells, with the parent drug only accounting for 1.4% (22 ng/mL) of the total drug, while the glucuronides accounted for 83.3% of the total and the hydrolysis product accounted for 15.3%. NU/ICRF 505 is stable in tissue culture over 24 h in the absence of cells.16 With increasing concentrations of propofol (ranging from 50–200 μM), there was a selective and progressive inhibition of glucuronidation by approximately 4-fold, resulting in an approximately 20-fold increase in the parent drug (from 22 ng/mL to 408 ng/mL). The formation of the hydrolysis product by esterase action was not inhibited by propofol and if anything there was an increase in its concentration caused by the increase in the parent drug. In conjunction with the inhibition of glucuronidation by propofol there was a 35-fold increase in the intracellular levels of NU/ICRF 505 determined by HPLC (Figure 3) from 5.2 ng/106 cells in the absence of propofol to 184 ng/106 cells in the presence of 200 μM. Only trace amounts of glucuronides were detected intracellularly because of their efficient cellular efflux by multidrug resistance transport proteins.
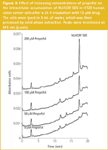
Figure 3
Effect of propofol on cellular accumulation of NU/ICRF 505 in HCT116 cells: By a combination of PCR and western blot analysis, HCT116 cells have been demonstrated not to express UGTs, and were used in the present study to control for non-selective effects of glucuronidation modulators on drug transport and metabolism. In the absence of glucuronidation, NU/ICRF 505 parent drug concentrations remained at a high level (874 ng/mL) in the tissue culture medium of HCT116 cells: 40-fold greater than in the tissue culture medium of HT29 cells (Figure 4, no propofol).
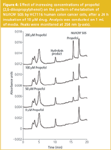
Figure 4
The intracellular uptake of NU/ICRF 505 into HCT116 was also concomitantly greater than in HT29 cells, with parent drug levels reaching a value of 2763 ng/106 cells almost 3 orders of magnitude greater than in HT29 cells, highlighting the enormous contribution of glucuronidation to drug clearance and drug resistance. Increasing concentrations of propofol (ranging from 50–200 μM) had little effect on the extracellular and intracellular concentrations of NU/ICRF 505, the former increased to 1268 ng/mL after 200 μM propofol because of a small effect on esterase-catalysed hydrolysis of the parent drug O-ethyl ester group to the free amino acid form (Figure 1).
These data show that propofol has little non-selective action on the cellular pharmacology of NU/ICRF 505 in the absence of glucuronidation and this conclusion is also supported by the observation that it has no significant effect on the growth inhibitory activity of the compound against HCT116 cells.4
The between day accuracy and precision for the determination of NU/ICRF 505 in tissue culture media and cell lysates was 91.8% ± 8.7 with the recovery of the solid-phase extraction technique being 90.2% ± 8.4.20
Modulation of SN-38 glucuronidation in HT29 cells by propofol: The analysis of SN–38 is complicated by the fact that the lactone ring of the drug (ring E, Figure 1) is chemically unstable and opens readily to yield a hydroxy acid (also known as the carboxylate form) at neutral and alkaline pH, but is stable at pHs below 5. We have previously reported the application of solid-phase extraction sample preparation to the simultaneous determination of the lactone and hydroxy acid forms of SN–38 in tissue media and cancer cells and described a set of conditions to almost eliminate in situ chemical degradation during sample preparation.18 These include storing samples in ice prior to extraction, using Tris-HCl buffer 7.4 throughout, maintaining the autosampler compartment at 4 °C and completing the analysis cycle in less than 4 h. At a concentration of 400 ng/mL of either the lactone or hydroxy acid — which is similar to the concentration of 1 μM/392 ng/mL used in the present study — the between day accuracy and precision of the methodology was 101.7% ± 2.2 for the lactone and 95.8% ± 3.7 for the hydroxy acid.18 Interestingly, lactone ring opening results in a 3-fold increase in peak area at the fluorescence wavelengths adopted in the present study.
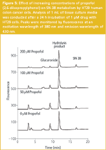
Figure 5
Analysis of the tissue culture media after a 24 h incubation of SN-38 with HT29 cells, in the absence of propofol, generated the following profile: SN–38 lactone, 87 ng/mL; SN–38 hydroxy acid, 245 ng/mL and SN–38 glucuronide 66 ng/mL (Figure 5). These data show two important observations. First, glucuronidation only accounts for 17% of the drug. Second, hydrolysis is the major pathway of biotransformation in tissue culture representing 62% of the drug. Nonetheless, increasing concentrations of propofol enabled the glucuronidation component to be inhibited effectively by over 80%, as in NU/ICRF 505, with only 11 ng/mL of the glucuronide metabolite in culture media after 200 μM of propofol. However, this large inhibition did not result in a concomitant rise in the active form of the drug the SN–38 lactone, which only increased to 103 ng/mL after 200 μM propofol. This was because of an increase also in the hydrolysis product to 304 ng/mL. The small increase in the extracellular concentration of lactone was also reflected by a small increase in intracellular lactone.
Effect of a range of UGT aglycones on cellular accumulation of NU/ICRF 505 in HT29 cells: In a series of parallel studies, we have demonstrated that the reduction in anticancer drug glucuronidation achieved by propofol was accompanied by the formation of a glucuronide metabolite of propofol, and thus was probably caused by the competitive inhibition of UGT1A9. Using HT29 cells as the model biological system and SN–38 and NU/ICRF 505 as the model drugs, the HPLC methodology was applied to the analysis of a number of UGT aglycone substrates. These included ibuprofen and methyl 4-hydroxybenzoate (methyl paraben), the former being a proprietary over-the-counter (OTC) medicine, the latter being a well-known food additive, both of which would be more appropriate and safer modulators of glucuronidation in patients than the general anaesthetic propofol. The results of a study with NU/ICRF 505 are shown in Figure 6, where the ability to restore intracellular levels of the parent drug is presented. A range of efficacies was obtained with propofol being the most effective. More recent studies, however, have now identified UGT aglycones that are 10 times more effective than propofol.
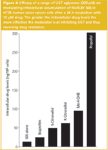
Figure 6
Conclusions
Methodology based on solid-phase extraction sample preparation and reversed-phase HPLC using Symmetry Shield as the stationary phase are presented that allow the simultaneous determination of two different anticancer drugs, their hydrolysis products and glucuronide metabolites in tissue culture media and cancer cells. This methodology thus allows modulation of drug glucuronidation to be monitored and potential modulators to be screened. The human colon cancer cell line HT29 is proposed to be a good in vitro biological model for such studies since it expresses a large number of different UGT isoforms. Highly effective and isoform selective inhibitors of glucuronidation have been identified using this methodology and cell line.
Jeffrey Cummings is a senior scientist responsible for quality control in the clinical and experimental pharmacology group of the Paterson Institute for Cancer Research, University of Manchester, UK. Dr Cummings has been involved in anticancer drug pharmacology for 25 years and has published over 140 papers in the fields of drug analysis, drug resistance and mechanism of drug action and presently sits on the editorial broad of the Journal of Chromatography B. Caroline Dive is the head of clinical and experimental pharmacology group of the Paterson Institute for Cancer Research. In addition, Professor Dive holds the chair of Pharmacy and Pharmacology at University of Manchester, UK.
References
1. M.M. Gottesman, Annu. Rev. Med., 53, 615–27 (2002).
2. G.D. Leonard et al., Curr. Opin. Investig. Drugs, 3, 1652–9 (2002).
3. J. Cummings et al., Biochem. Pharmacol., 63, 607–13 (2002).
4. J. Cummings et al., Cancer Res., 63, 8443–50 (2003).
5. J. Cummings et al., Biochem. Pharmacol., 67, 31–9 (2004).
6. J. Fassberg et al., J. Pharm. Sci., 81, 676–684 (1992).
7. K. Akimoto et al., Chem. Pharm. Bull, 42, 2135–2138 (1994).
8. W.J. Loos et al., J. Chromatogr. B, 678, 309–315 (1996).
9. D.L. Warner et al., J. Chromatogr. B Biomed. Sci. Appl., 691, 161–71 (1997).
10. N. Kaneda et al., Biol. Pharm. Bulletin, 20, 815–9 (1997).
11. J. Escoriaza et al., J. Chromatogr B Biomed. Sci. Appl., 740, 159–68 (2000).
12. D.F. Chollet et al., J. Chromatogr B Biomed. Sci. Appl., 718, 163–75 (1998).
13. S. Poujol et al., Clin. Chem., 49, 1900–8 (2003).
14. X. Yang et al., J. Chromatogr B Analyt. Technol. Biomed. Life Sci., 821, 221–8 (2005).
15. L.P. Rivory et al., J. Chromatogr. B Biomed. Sci. Appl., 714, 355–9 (1998).
16. J. Cummings et al., Anticancer Drugs, 11, 367–382 (1996).
17. J. Cummings et al., J. Chromatogr. B, 685, 159–164 (1996).
18. G. Boyd et al., Anal. Biochem., 297, 15–24 (2001).
19. J. Cummings et al., Cancer Chemother. Pharmacol., 37, 103–109 (1995).
20. J. Cummings et al., Chromatographia, 55 (S157–S163 (2002).
21. R.H. Tukey et al., Mol Pharmacol., 59, 405–414 (2001).
22. P.I. Mackenzie et al., Pharmacogenet. Genomics, 15, 677–85 (2005).
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.
Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.