Extraction of 14 Benzodiazepines by a Supported Liquid Extraction Plate, ISOLUTE SLE+
Benzodiazepines are commonly assayed to determine therapeutic dose levels and for forensic investigation. These drugs are commonly prescribed to alleviate pain and therapeutic dosing needs to be monitored.
Lee Williams, Rhys Jones, Steve Jordan, Richard Calverley, Claire Desbrow, Gary Dowthwaite, and Elena Gairloch, Biotage GB Limited
Benzodiazepines are commonly assayed to determine therapeutic dose levels and for forensic investigation. These drugs are commonly prescribed to alleviate pain and therapeutic dosing needs to be monitored. Additionally, the use of this class of drugs in "date rape" cases has lead to a need for forensic monitoring. Biotage have successfully applied supported liquid extraction, utilizing ISOLUTE® SLE+, to analyze 14 benzodiazepines by LC–MS from whole blood, plasma, and urine. These procedures are fully automation compatible, dramatically reducing cost and increasing throughput.
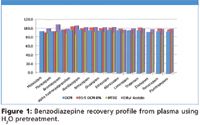
Figure 1
Method
ISOLUTE SLE+ Supported Liquid Extraction Plate, 200 μL sample size, part number 820-0200-P01, was used. Blank matrix (100 μL) was spiked with the benzodiazepines at 50 ng/mL. The matrix was then diluted 1:1 v/v with H2O prior to loading. This sample dilution results in an approximate loading pH of 8.0. For the whole blood cell lysis, samples were sonicated for 10 min in buffer, followed by centrifugation at 11,000 rpm for 10 min and the cellular debris discarded. 200 μL of pretreated plasma was applied to the plate and a pulse of vacuum applied to initiate flow. The samples were then left to absorb for 5 min. Analytes were eluted by the addition of 1 mL of various water immiscible extraction solvents. The extraction solvents evaluated were DCM, 95:5 (v/v) DCM/IPA, MTBE, and EtOAc. The eluate was then evaporated to dryness and the analytes reconstituted in 500 μL of 80:20 (v/v) H2O/MeOH prior to analysis.
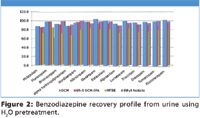
Figure 2
Results
Recoveries of greater than 80% were observed for the majority of analytes from all three matrices using various extraction solvents. RSDs were below 10%, demonstrating reproducible extraction and recoveries. Pretreatment with 1% formic acid, 0.1% formic acid, H2O and 0.5M NH4OH for plasma and urine and 0.1% formic acid and H2O for whole blood were also investigated (data not shown, available upon request). These pretreatment conditions yielded a range from pH 3.2–10.4. Consistently high recoveries were observed using H2O pretreatment of plasma, urine, and blood for all extraction solvents (Figures 1–3, respectively). Similar, though slightly attenuated, recoveries were obtained from all analytes across the full pretreatment pH range (pH 3.2–10.4). These results allow for the use of a broad range of buffer conditions to be used at a pH compatible with other analytes of interest. This easily automatable methodology allows for high recoveries of a broad range of analytes using various buffers and extraction solvents.
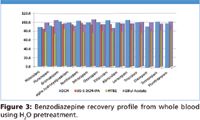
Figure 3

Biotage, LLC
10430 Harris Oaks Blvd., Suite C, Charlotte, NC 28269
Website: www.biotage.com
Email: product_info@biotage.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













