Exploiting the Tunability and Versatility of Ionic Liquids as Extraction Phases for Targeted and Omics Studies in the Plant and Food Fields
Natural product and food analyses must cover a wide range of topics, from genomics and metabolomics studies to quality control and quantification of endogenous and exogenous compounds, often for safety purposes. In both cases, the choice of sample preparation procedure plays a fundamental role. For this range of analyses, ionic liquids (ILs) are a very versatile tool: By combining moieties with different functional group, ILs can be tuned to obtain extraction media with different properties and selectivity.
Food and natural product analyses must cover a wide range of topics, ranging from genomic sand metabolomics studies to quality and safety controls. In all cases, sample preparation plays a fundamental role (1). Indeed, omics studies require that as many metabolites as possible be extracted (2), while still taking into consideration that a universal extraction phase or technique is not realistic in everyday practice because of the complexity of the natural systems and the chemical diversity of compounds. On the other hand, quality control analyses require the determination of a single or multiple metabolites, often present at trace levels in the samples, and the extraction method must therefore recover target compounds at very low limits, often in agreement with regulations, meaning that a high enrichment rate is necessary. In both cases, the choice of sample preparation strategy must also take into account compatibility with the downstream analytical system, which is commonly gas chromatography (GC) for the analysis of the most volatile analytes, or high performance liquid chromatography (HPLC) for nonvolatiles.
Ionic liquids (ILs) are a very interesting class of molten salts whose physicochemical properties have inspired the rapid development of new applications in analytical chemistry and in the sample preparation field (3). One of the main features of ILs, compared to traditional organic solvents, is their ability to incorporate, within their chemical structure, different functional groups that can influence their physico-chemical and solvation properties, enabling them to interact selectively with specific analytes or classes of analytes. In addition, because they have negligible vapor pressure at room temperature, they prevent solvent loss to the atmosphere, thus decreasing the environmental footprint. Their ability to dissolve cellulose and other cell-wall components, such as hemicellulose and lignin, is a further important feature of ILs, in particular when dealing with samples of vegetable origin. Indeed, plant cells are protected by thick and robust lignocellulosic walls that must be disrupted if an efficient extraction of intracellular metabolites is to be achieved. Many studies have demonstrated that ILs can favor the decrystallization of the cellulose portion of the cell walls and the simultaneous disruption of the lignin and hemicellulose network (4). This ability can be exploited, for instance, in the pretreatment of plant material submitted to extraction or distillation (5).
The aforementioned properties have allowed ILs to find many applications as solvents and sorbents in extractions of bioactive compounds from food and natural products (1). When used as solvents, ILs can be adopted either in simple liquid– liquid or solid–liquid extractions (LLE/SLE) or in microwave-assisted (MA) and ultrasound-assisted (UA) extractions. In addition, their varying solvation properties allow them to be applied in different (micro) extraction modes, exploiting also the ability to tune their solubility in water. ILs can also incorporate a paramagnetic element into their chemical structure, either in the cation or in the anion, giving rise to magnetic ionic liquids (MILs), a subclass of ILs whose application in sample preparation has rapidly grown. These compounds have great potential in the extraction of endogenous and exogenous metabolites from natural matrices because they combine all the standard features of ILs with paramagnetic characteristics, allowing them to be easily separated using an external magnetic field, avoiding tedious centrifugation and filtration steps. Finally, ILs can also be incorporated into solid-phase platforms, either alone, as polymeric ionic liquids (PILs), or in combination with other sorbents (such as natural sorbents, molecular imprinted polymers, and carbon-based materials) to form composites that exploit the unique features of ILs (6).
The use of ILs as extraction phases has been integrated into different types of omics studies, from genomics (with the extraction of nucleic acids) (7) to metabolomics (1). In metabolomics studies, ILs can be exploited in the simultaneous determination of volatile and nonvolatile components or of hydrophilic and hydrophobic nonvolatile compounds (8).
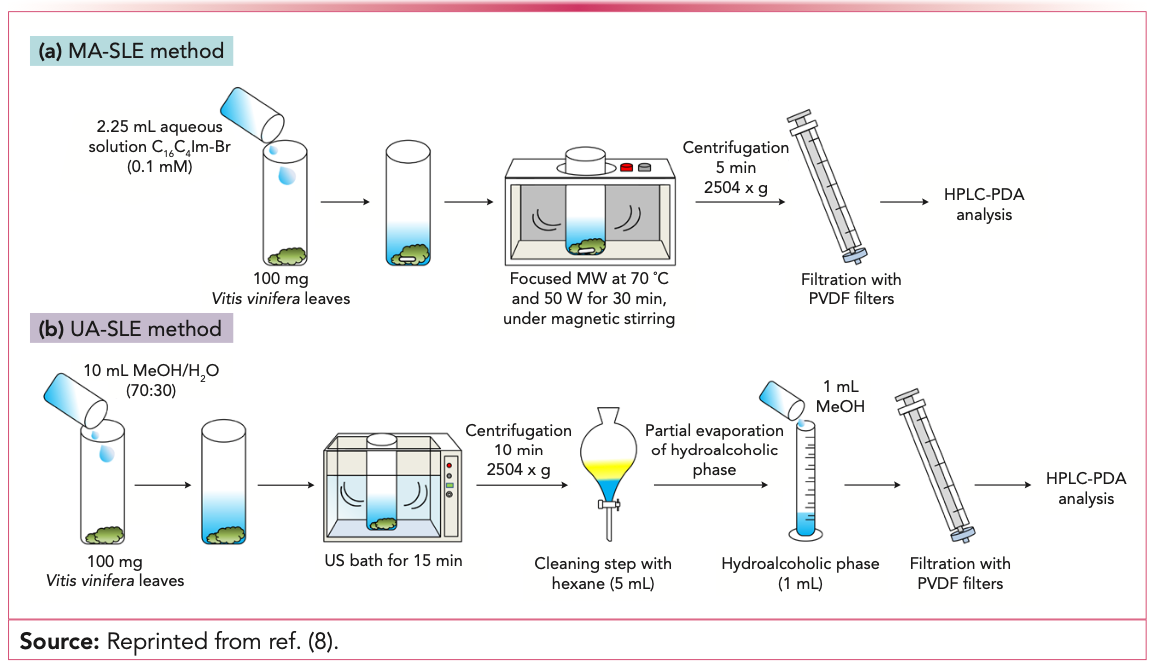
In the context of metabolomics studies, ILs can be useful not only to increase the array of extracted metabolites but also to improve sustainability. In this regard, ILs have been exploited as surfactants for the extraction of bioactive phenolic compounds from grape (Vitis vinifera L.) leaves (9). The study developed a green alternative to the conventional ultrasound-assisted extraction method reported in literature that, although effective in the extraction of the target analytes, requires multiple steps and the consumption of a high amount toxic organic solvents. Different IL-based surfactants were evaluated, and the method was optimized, resulting in the use of small amounts of sample and extraction phase, low concentrations of the selected 1-hexadecyl-3-butyl imidazolium bromide IL, and only 30 min of extraction time. Figure 1 reports the comparison of the conventional and IL-based extraction method, showing that the proposed protocol is highly sustainable, as it does not require organic solvents or a clean-up step, apart from filtration before injection into the analytical HPLC system. In addition, it provides optimal quantification performance and better extraction efficiency than the conventional method. The proposed extraction protocol was applied for the determination of the polyphenolic pattern of different varieties of grape leaves from the Canary Islands, using HPLC and photodiode array detection for quantification.
FIGURE 1: Scheme of two extraction procedures of grape leaves: (a) IL-based MA-SLE and (b) conventional UA-SLE. Reprinted from ref. (8).
On the other hand, the chemical structure of ILs can be tuned for selective recovery of metabolites belonging to specific chemical classes. In the food and natural products field, this feature can be exploited for determining markers of quality or health concerns.
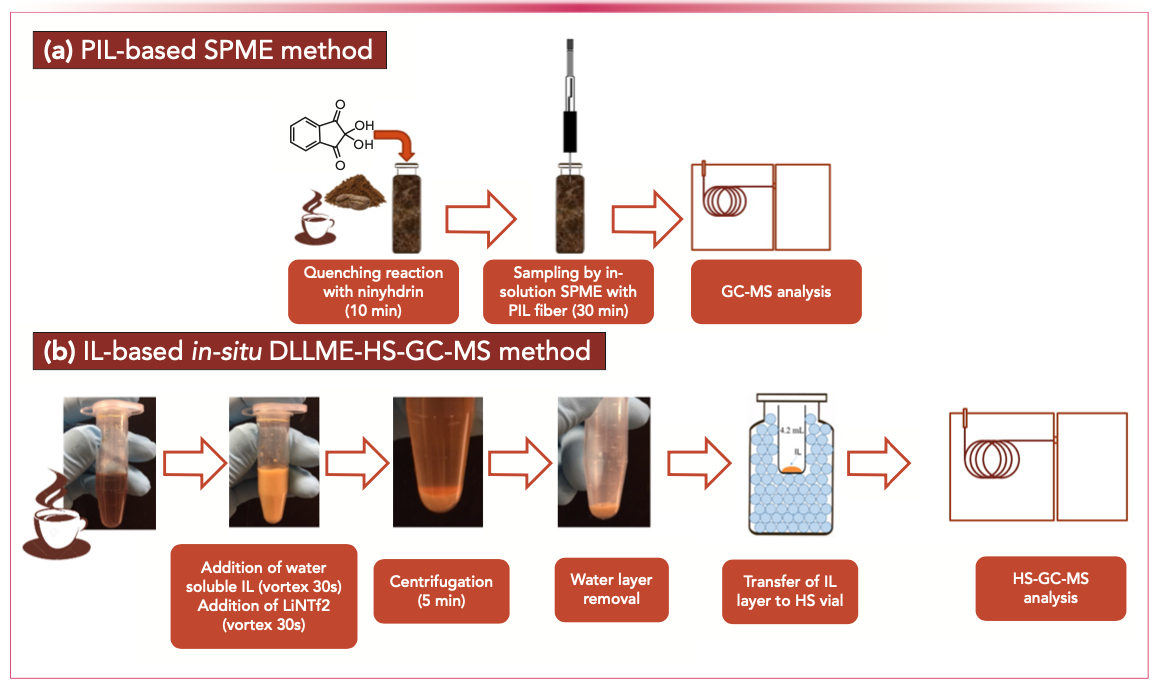
As an example, the selectivity of ILs was successfully exploited for the extraction and determination of acrylamide from coffee (10–12). Acrylamide is an unsaturated amide formed when carbohydrate-rich foods are subjected to high temperatures during cooking or thermal processing. Several studies have revealed that acrylamide has serious toxicological properties, and therefore it has been classified as a possible carcinogen for humans. Regulatory agencies have set benchmark levels that food business operators must use as reference for their products. Acrylamide analysis is a considerable challenge because of both its chemical characteristics and trace concentration. Established ISO methods adopt HPLC with tandem mass spectrometry (HPLC–MS/MS) or GC–MS after derivatization, and, in both cases, sample preparation includes a large number of steps (protein precipitation and consecutive steps of SPE) and requires a high number of chemicals and single-use consumables, thus resulting in a methodology that is time-consuming and unsustainable, and which cannot easily be automated. As an alternative, direct immersion solid-phase microextraction (SPME) combined with GC–MS using fibers coated with PIL sorbents can be used to recover acrylamide with a very high selectivity (10). The method is effective, provided that a very simple quenching reaction using ninhydrin is carried out to degrade free asparagine before the extraction step and to inhibit the formation of additional acrylamide at the high temperatures of the GC inlet. In addition, tailoring of the monomer and crosslinker structures of the PIL has made it possible to design derivatives with better selectivity for acrylamide and to achieve a limit of quantitation (LOQ) for acrylamide in the ppt range (11). The matrix compatibility of the PIL sorbent coatings was proved by analyzing several coffee powders and brew samples, achieving LOQ values comparable to those of the ISO method. The PIL-based SPME method is more sustainable than the official method, can be fully automated, and involves the use of conventional instrumentations. The proposed protocol is also quicker than the original one, although it still requires 30 min of sampling and fiber reconditioning to avoid carryover effects. Further improvements in this respect have been obtained with the development of an in situ dispersive liquid–liquid microextraction (DLLME) method coupled to headspace (HS) GC–MS (12). In this case, fine droplets of hydrophobic ILs, obtained by submitting halide-based ILs to metathesis reaction with lithium bis[(trifluoromethyl) sulfonyl]imide, have been used to extract acrylamide from coffee brews in a few seconds. After centrifugation, the acrylamide-enriched IL droplet can be recovered and analyzed by HS–GC-MS. The proposed method showed good analytical precision and matrix compatibility for coffee brews, and provided detection limits down to the low ppb level, showing a further reduction of pretreatment and extraction times and the elimination of the possible carryover effect compared to the SPME method. Figure 2 reports the schemes of the IL-based sample preparation methods for the determining acrylamide in coffee samples.
FIGURE 2: Scheme of IL-based sample preparation methods for the determination of acrylamide in coffee samples: (a) PIL-based SPME and (b) in-situ DLLME-HS-GC-MS.
Outlook
Despite the clear advantages of IL-based extractions, these molecules also have some limitations. Although they were originally considered to be green chemicals, some concerns regarding IL toxicity and potential environmental impact have started to arise in recent years because most of them are obtained from fossil sources and show poor degradability. In this respect, current trends are focused on designing more degradable and less toxic ILs by using, for instance, ILs with fluorine-free anions and with more biodegradable cations (such as pyridinium or guanidinium) or with shorter IL side chains in any of the moieties. However, these derivatives still require a large amount of non-renewable solvents for their synthesis. The use of anions and cations that are fully or partly derived from natural compounds would therefore be an ideal and sustainable approach for the development of a new generation of bio-renewable ILs (bio-ILs) (13). Unfortunately, these bio-ILs have seldom been applied in sample preparation and future advances are thus expected, as well as their application in the food and natural product fields. The full potential of ILs in sample preparation can be therefore further explored—the systematic study and the exploitation of the almost infinite number of different combinations of cations and anions opens up possibilities for the development of ever new unconventional and environmentally friendly approaches, in particular in the field of microextractions.
References
(1) J.L. Wolfender, S. Rudaz, Y.H. Choi, and H.K. Kim, Curr. Med. Chem. 20, 1056–1090 (2013). https://doi.org/10.2174/092986713805288932
(2) G. Mastellone, A. Marengo, B. Sgorbini, P. Rubiolo, and C. Cagliero, TrAC Trends Anal. Chem. 141, 116288 (2021). https://doi.org/10.1016/j.trac.2021.116288
(3) M.J. Trujillo-Rodríguez, H. Nan, M. Varona, M.N. Emaus, I.D. Souza, and J.L. Anderson, Anal. Chem. 91, 505–531 (2019). https://doi.org/10.1021/acs.analchem.8b04710
(4) A. Brandt, J. Gräsvik, J.P. Hallett, and T. Welton, Green Chem. 15, 550–583 (2013). https://doi.org/10.1039/c2gc36364j
(5) J. Jiao, D.-H. Ma, Q.-Y. Gai, W. Wang, M. Luo, Y.-J. Fu, and W. Ma, Anal Chim. Acta 804, 143–150, 2013. https://doi.org/10.1016/j.aca.2013.10.035.
(6) A. Gutiérrez-Serpa, P.I. Napolitano-Tabares, J. Šulc, I. Pacheco-Fernández, and V. Pino, Separations 7, 1–32 (2020). https://doi.org/10.3390/separations7030037
(7) A. Marengo, C. Cagliero, B. Sgorbini, J.L. Anderson, M.N. Emaus, C. Bicchi, C.M. Bertea, and P. Rubiolo, Plant Methods 15, 23 (2019). https://doi.org/10.1186/s13007019-0408-x
(8) T. Wang, Q. Wang, P. Li, and H. Yang, Green Chem. 21, 4133–4142 (2019). https://doi.org/10.1039/c9gc00995g.
(9) G. Mastellone, I. Pacheco-Fernandez, P. Rubiolo, V. Pino, and C. Cagliero, Molecules 25, 3072 (2020). https://doi.org/10.3390/molecules25133072
(10) C. Cagliero, T.D. Ho, C. Zhang, C. Bicchi, and J.L. Anderson, J. Chromatogr. A 1449, 2–7 (2016). http://dx.doi.org/10.1016/j.chroma.2016.04.034
(11) C. Cagliero, H. Nan, C. Bicchi, and J.L. Anderson, J. Chromatogr. A 1459, 17–23 (2016). http://dx.doi.org/10.1016/j.chroma.2016.06.075
(12) C. Zhang, C. Cagliero, S.A. Pierson, and J.L. Anderson, J. Chromatogr. A 1481, 1–11 (2017). https://doi.org/10.1016/j.chroma.2016.12.013
(13) J. Hulsbosch, D.E. De Vos, K. Binnemans, and R. Ameloot, ACS Sustain. Chem. Eng. 4, 2917–2931 (2016). https://doi.org/10.1021/acssuschemeng.6b00553.
Cecilia Cagliero is an associate professor in the Department of Pharmaceutical Science and Technology at the University of Turin, in Turin, Italy. Direct correspondence to: cecilia.cagliero@unito.it

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.









