Innovations and Strategies of Sample Preparation Techniques to Reduce Matrix Effects During LC–MS/MS Bioanalysis
Bioanalysis quantitatively measures xenobiotics and biotics in biological systems. In biological matrixes, the target analytes coexist with much higher concentrations of exogenous and endogenous compounds (for example, metabolites of the target analyte, proteins, or phospholipids) whose chemical structures resemble the structures of the analytes. Liquid chromatography– tandem mass spectrometry (LC–MS/MS) has become the technique of choice for bioanalysis. Ion suppression is a form of matrix effect that impacts LC–MS techniques, regardless of the sensitivity or selectivity of the mass analyzer. In general, improving sample preparation is the most effective way of circumventing ion suppression. This article describes innovations and strategies of sample preparation techniques (protein precipitation, liquid-liquid extraction, or solid-phase extraction) to reduce matrix effects during LC–MS/MS analyses.
Bioanalysis quantitatively measures xenobiotics and biotics in biological systems. In biological matrixes, the target analytes coexist with much higher concentrations of exogenous and endogenous compounds (for example, metabolites of the target analyte, proteins, or phospholipids) whose chemical structures resemble the structures of the analytes.
Liquid chromatography–tandem mass spectrometry (LC–MS/MS) has become the reference technique for bioanalysis because of its high sensitivity and selectivity. The presence of endogenous compounds in biological samples can enhance or suppress the target ion signal during LC–MS/ MS analysis, with ion suppression being the most common matrix effect. Phospholipids are the major cause of ion suppression in regards to analysis of samples extracted from biological tissues or plasma samples (1). Regarding atmospheric pressure ionization techniques, atmospheric pressure chemical ionization (APCI) and electrospray ionization (ESI) have been the most frequently employed during LC–MS/MS analysis. ESI is the most susceptible to ion suppression (2,3).
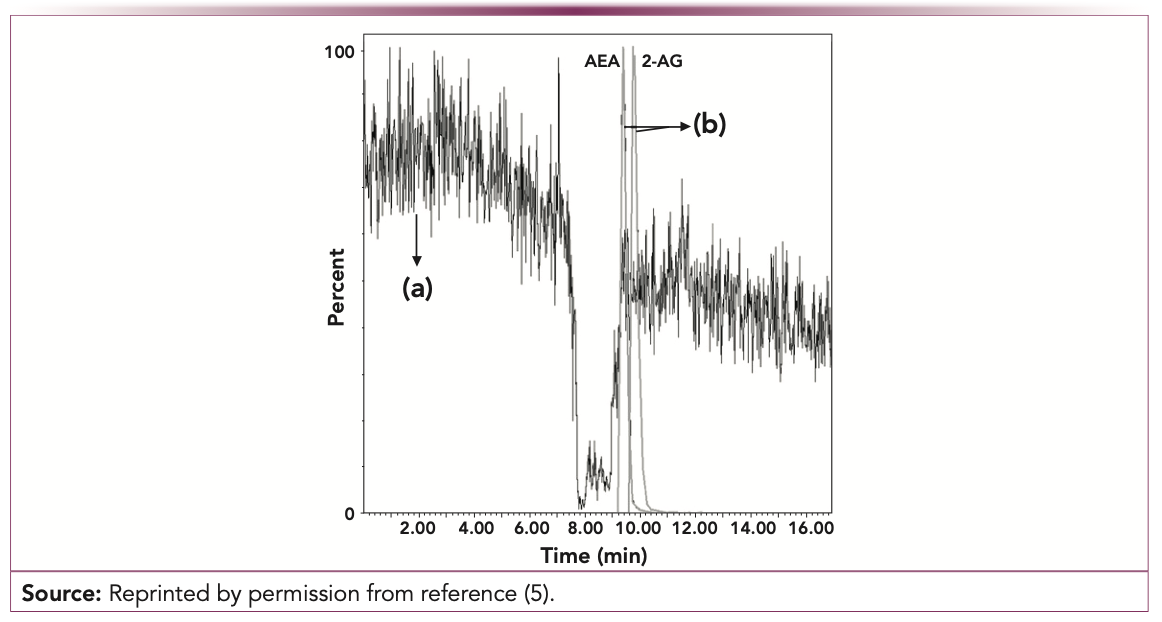
There are two established methods to assess matrix effects—the qualitative post-column infusion method (4,5), and the post-extraction spike method (6), that can assess matrix effects quantitatively (see Figure 1). Although matrix effects cannot be completely avoided during LC–MS analysis, they can be compensated within an assay by using an appropriate internal standard (IS). The IS will be subject to the same degree of ion suppression or enhancement as the target analyte. If available, a stable isotope-labeled IS (SIL-IS) is preferred, given that the SIL-IS will have almost identical elution characteristics as their non-labeled counterpart, but they might be fallible (7).
FIGURE 1: Post-column infusion chromatogram of anandamide (AEA) and 2-arachidonoyl glycerol (2-AG) for the column switching UHPLC–MS/MS method of (a) ionization suppression of a blank plasma sample, and (b) AEA and 2-AG standard solution at a concentration of 0.3 ng/mL for AEA and 0.12 ng/mL for 2-AG.
Even though an SIL-IS may compensate for matrix effects, sensitivity loss because of such effects may not be overcome.
Apart from using an appropriate IS, LC and MS conditions can be manipulated, and sample preparation procedures can be optimized to eliminate matrix interferences. In general, improving sample preparation is the most effective way of circumventing ion suppression. Conventional sample preparation techniques for LC–MS analysis are protein precipitation (PPT), liquid-liquid extraction (LLE), or solid-phase extraction (SPE).
Protein Precipitation
Common protein precipitants include: water-miscible organic solvents (efficiency order: acetonitrile > acetone > ethanol > methanol); acids (trichloroacetic acid [TCA] [5–15% TCA] and perchloric acid [PCA] [6% PCA]); metal ions; and salts. Among these precipitants, water-miscible organic solvents and acids are most often applied.
Acetonitrile, TCA, and zinc sulfate are optimal at removing protein in their categories (>96, 92, and 91% protein precipitation efficiency at a 2:1 ratio of precipitant to plasma, respectively) (8).
An automated (high-throughput) option is a 96-well protein precipitation filter plate (9).
The advantages of PPT are simplicity; minimal loss of sample; inexpensive reagents; applicability to a wide range of analytes; and easy automation. Disadvantages of PPT include inability to concentrate analytes; and significant ion suppression, caused mostly by phospholipids. After PPT, an acetonitrile extract contained fewer phospholipids (~50% in total) than a methanol extract (10). To decrease the effect of phospholipids, the super- natant or filtrate solution (through a 10,000-Da membrane within the plate) should be diluted (40-fold) with the mobile phase before PPT. The dilution approach is effective, easy to perform, and fast when it does not affect the required sensitivity of the method (10,11).
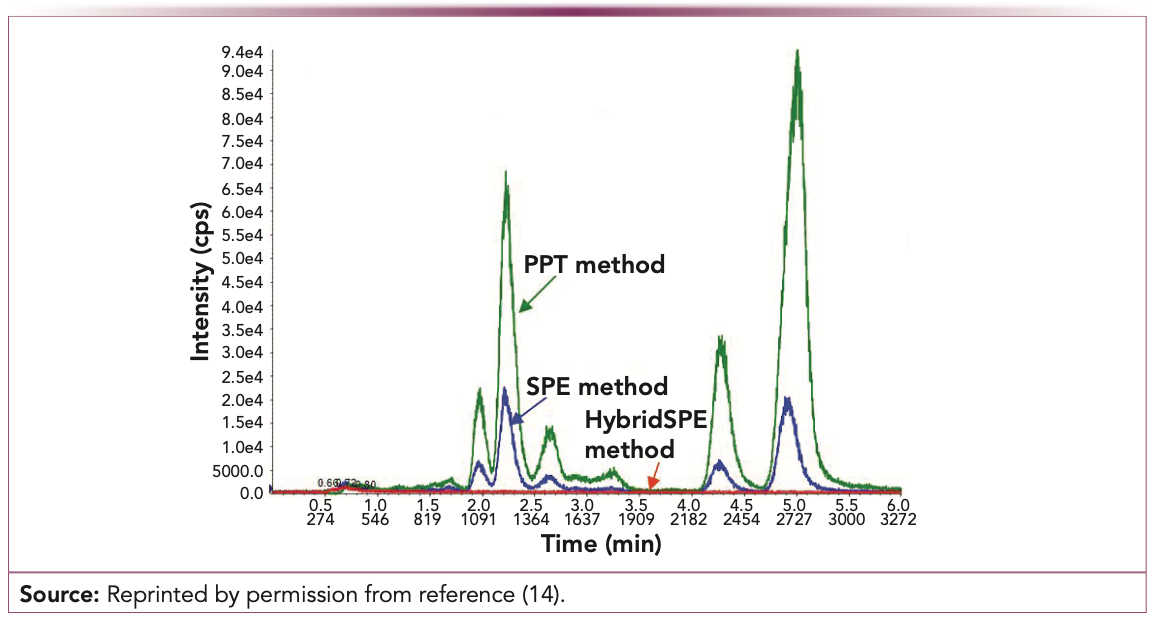
Recently, PPT plates that are packed with materials (zirconium-coated silica) that specifically retain phospholipids have been developed (see Figure 2) (12–14).
FIGURE 2: Phospholipids matrix interference in different sample preparation techniques. HybridSPE is the new zirconium-coated silica sorbent applied to eliminate interference from phospholipids.
Liquid-Liquid Extraction (LLE)
LLE can be accomplished by using a single immiscible organic solvent (for example, n-hexane heptane, dichloromethane, ethyl acetate, methyl tert-butyl ether, or diethyl ether) or solvent mixtures to improve the sample preparation efficiency or to minimize matrix effects. Generally, alcohols (such as 1-propanol or 1-butanol) or acetonitrile are added to relatively nonpolar solvents.
The pH of the aqueous matrix should be adjusted two pH units higher than the pKa of a basic analyte, or two pH units lower than the pKa of an acidic analyte, so that 99% of the analyte will be uncharged. The use of an acidic or basic pH is highly recommended to prevent impurities, such as phospholipids and cholesterol esters, from being extracted (15).
Double LLE has been used to improve assay selectivity, or to minimize matrix effects. Initially, hydrophobic endogenous interferences are usually extracted by highly non-polar solvents (for example, hexane), while the analyte remains in the sample matrix. After the hexane layer is removed and discarded, the aqueous phase is further extracted by using a moderately nonpolar solvent (for example, methyl tert-butyl ether or ethyl acetate) (16,17).
Salting-out assisted LLE (SALLE) is an alternative type of LLE that has been employed during LC–MS bioanalysis (18). Compared to conventional LLE, SALLE has broader application, and covers a range from low to highly lipophilic molecules while providing better analyte recovery. One apparent drawback of SALLE is the higher matrix effect as compared to conventional LLE; this is because SALLE extracts tend to contain more endogenous compounds.
Restricted access–volatile supramolecular solvents (RAM–VOL–SUPRAS) have been proposed as a new strategy to remove protein and phospholipids rapidly, and to extract analytes efficiently during LC–MS bioanalysis (19).
Solid-Phase Extraction
SPE, whether manual, automated, or online with LC–MS systems, selectively preconcentrates (10–100-fold enrichment) the target analytes or isolates the interfering biological matrix components (for example, the hybrid zirconia-silica phase with phospholipids). Selective stationary phases can retain the target analytes on the basis of ionic or hydrogen bonding, or dipole–dipole, dipole–induced dipole, or dispersion forces.
Different SPE polymeric phases (mixed-mode cation exchange, reversed-phase, and cation exchange) have been evaluated to minimize the effect of phospholipids from plasma samples during LC–MS/MS analysis. Polymeric mixed-mode strong cation exchange combining reversed-phase and ion exchange mechanisms have yielded the best results (20).
Given the strong retention of phospholipids on reversed-phase sorbents, the target analyte can be selectively eluted during SPE by using a partially aqueous elution solvent (21).
The stationary phase selectivity can be enhanced by employing immunosorbent and molecularly imprinted polymer (MIP) via specific molecular recognition, which reduces matrix effects. In this scenario, restricted-access materials (RAM) by physical and chemical diffusion barriers prevent large interfering molecules such as proteins and phospholipids from being retained (22). Hybrid materials (23) are promising sorbents. RAM–MIPs perfectly combine the advantages of RAM and MIPs, improving the selectivity of target small molecules, while the hydrophilic surface excludes endogenous compounds of the biological samples (24). Combining different platforms (for example, PPT/LLE [25], PPT/SPE [26], PPT/SALLE [27], SPE/DLLME [28], or LLE/SPE [29]) is useful to decrease matrix effects.
Current Developments and Future Trends
Recently, sample preparation techniques have been geared toward miniaturization, development of selective new sorbent materials, and high-throughput performance with online coupling to analytical instruments. A miniaturized system requires a smaller amount of sample and organic solvent, and an online system condenses the sample preparation steps (thereby reducing manual error and improving accuracy for repeated trials), while decreasing analysis time and costs. In this context, online capillary solid-phase microextraction (in-tube SPME) coupled to high-performance liquid chromatography (HPLC) is worthy of mention (30,31).
Future perspectives include online coupling of miniaturized sample preparation techniques with miniaturized chromatographic devices (such as capillary-LC and nanoLC). This combination may result in more cost-effective, sensitive, and sustainable methods that will significantly impact pharmaceutical or clinical analyses of biofluids (32).
Acknowledgments
The authors would like to acknowledge Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), processes, 2019/19485–0, 2017/02147–0, and 2020/00126–8) and Instituto Nacional de Ciência e Tecnologia Translacional em Medicina (INCT-TM) (465458/2014–9) for financial support and fellowships.
References
(1) O.A. Ismaiel, T. Zhang, R.G. Jenkins, and H.T. Karnes, J. Chromatogr. B 878(31), 3303–3316 (2010).
(2) X. Xu et al., Rapid Commun. Mass Spectrom. 19(18), 2643–2650 (2005).
(3) D.A. Volmer and L.L. Jessome, LCGC North Am. 24(5), 498–510 (2006).
(4) R. Bonfiglio, R.C. King, T.V. Olah, and K. Merkle, Rapid Commun. Mass Spectrom. 13(12), 1175–1185 (1999).
(5) C. Marchioni et al., Anal. Bioanal. Chem. 409(14), 3587–3596 (2017).
(6) B.K. Matuszewski, M.L. Constanzer, and C.M. Chavez-Eng, Anal. Chem. 75(13), 3019–3030 (2003).
(7) E. Stokvis, H. Rosing, and J. H. Beijnen, Rapid Commun. Mass Spectrom. 19(3), 401–407 (2005).
(8) C. Polson, P. Sarkar, B. Incledon, V. Raguvaran, and R. Grant, J. Chromatogr. B 785(2), 263–275 (2003).
(9) T. Margaryan et al., Biomed. Chromatogr. 34(8) (2020).
(10) D. Neville, R. Houghton, and S. Garrett, Bioanalysis 4(7), 795–807 (2012).
(11) Z. Mao et al., Anal. Bioanal. Chem. 409(11), 3025–3032 (2017).
(12) M. Rahman et al., J. Pharm. Bioallied Sci. 4(4), 267 (2012).
(13) V. Pucci, S. Di Palma, A. Alfieri, F. Bonelli, and E. Monteagudo, J. Pharm. Biomed. Anal. 50(5), 867–871 (2009).
(14) C. Mi, C. Aurand, A. Trinh, and M. Ye, “High Throughput Removal of Both Phospholipids and Proteins in Bioanalytical Sample Preparation” (Supelco [Division of Sigma-Aldrich], Bellefonte, Pennsylvania, at <chrome-extension://oemmnd- cbldboiebfnladdacbdfmadadm/ https://www.sigmaaldrich.com/ deepweb/assets/sigmaaldrich/marketing/global/documents/947/310/ t409038h.pdf>
(15) P.B. Kyle in Mass Spectrometry for the Clinical Laboratory, Hari Nair and William Clarke, Eds. (Elsevier, London, United Kingdom, 2017) pp. 131–163. doi:10.1016/ B978-0-12-800871-3.00007-9
(16) J. Hou, F. Qu, C. Wu, Q. Ren, and J. Zhang, J. Pharm. Biomed. Anal. 66, 232– 239 (2012).
(17) C. Domenech-Coca et al., J. Chromatogr. B 1108, 11–16 (2019).
(18) I. G. C. Oliveira and M. E. C. Queiroz, J. Chromatogr. B 1158, 122351 (2020).
(19) J.Á. Salatti-Dorado, N. Caballero-Casero, M.D. Sicilia, M.L. Lunar, and S. Rubio, Anal. Chim. Acta 950, 71–79 (2017).
(20) E. Chambers, D.M. Wagrowski-Diehl, Z. Lu, and J.R. Mazzeo, J. Chromatogr. B 852(1–2), 22–34 (2007).
(21) M. Lahaie, J.-N. Mess, M. Furtado, and F. Garofolo, Bioanalysis 2(6), 1011–1021 (2010).
(22) S. Souverain, S. Rudaz, and J.-L. Veuthey, J. Chromatogr. B 801(2), 141–156 (2004).
(23) N. Fontanals, R.M. Marcé, and F. Borrull, Separations 6(4), 56 (2019).
(24) H. He et al., Anal. Bioanal. Chem. 407(2), 509–519 (2015).
(25) Q. Wang et al., Bioanalysis 7(7), 885–894 (2015).
(26) Y.-J. Xue, J.B. Akinsanya, J. Liu, and S.E. Unger, Rapid Commun. Mass Spectrom. 20(18), 2660–2668 (2006).
(27) F. Myasein, E. Kim, J. Zhang, H. Wu, and T. A. El-Shourbagy, Anal. Chim. Acta 651(1), 112–116 (2009).
(28) F. Fanti et al., J. Pharm. Biomed. Anal. 186, 113302 (2020).
(29) A.W. Taylor and M.G. Traber, Anal. Biochem. 396(2), 319–321 (2010).
(30) C.F. Grecco, I.D. Souza, and M.E.C. Queiroz, J. Sep. Sci. 44(8), 1662–1693 (2021).
(31) M.E. Costa Queiroz, I. Donizeti de Souza, and C. Marchioni, TrAC Trends Anal. Chem. 111, 261–278 (2019).
(32) H.D. Ponce-Rodríguez, J. Verdú-Andrés, R. Herráez-Hernández, and P. Campíns-Falcó, Molecules 25(10), 2460 (2020).
Maria Eugênia C. Queiroz is a professor of chemistry at the University of São Paulo, in São Paulo, Brazil. Israel D. Souza is a PhD researcher at the University of São Paulo under the supervision of Dr. Queiroz. Direct correspondence to: mariaeqn@ffclrp.usp.br

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.













