Microbial Metabolomics Based on GC Analysis as the New Avenue in Understanding the Complex Systems of Microorganisms: Current Challenges on Sample Preparation
Microbial metabolomics includes a set of analytical tools that have been gaining enormous relevance because of the amount of data generated and the information that has been made available. Because of the wide coverage of microbial metabolomics, increased focus has emerged regarding sample preparation of microbial metabolomics before chromatographic analysis. One challenging issue on constructing microbial metabolomics workflows and several case-studies were used to illustrate the main challenges and new achievements in this field of study.
Microorganisms are found everywhere in the environment and play an important role in various processes. Pathogenic microorganisms can cause diseases in hosts during interactions or provoke damage and off-flavors in a wide variety of foods (1). The virulence factors aggravate the defense mechanisms of the host to establish infections, and the nature and type of virulence factors determine the pathogenicity of microorganisms. For instance, Staphylococcal food poisoning is a disease that results in significant health and economic losses and is caused by Staphylococcus aureus (S. aureus) strains, which are able to produce enterotoxins. Recent studies revealed that S. aureus enterotoxic and non-enterotoxic strains may be distinguished based on their volatile metabolites’ profiles (1). Otherwise, microorganisms can therefore be seen as suppliers of so-called ecosystem services, which are fundamental to the environment, human life, and industrial activities (2–5). By studying the complete set of metabolites within a microorganism and monitoring the global outcome of inter- actions between its development processes and the environment, it might be possible to provide a more accurate snapshot of the physiological state of the cell and the phenomena that modulate its metabolic pathways (6).
Microbial metabolomics is a comprehensive analysis of metabolites within a microorganism that may help to better understand complex biological systems and supports a new approach in microbial research. The microorganisms produce a wide range of metabolic products, which are the final products of biochemical processes and a result of environmental and genetic interactions, such as volatile metabolites, that can be used as unique volatile metabolic fingerprints of each species, and possibly of strains. Metabolomic profiling may provide additional information about the processes affected by specific microorganisms or microbiota alterations. Metabolomic profiling may also provide information about how the environment affects organisms that are susceptible to environmental changes and stress conditions. This data is particularly relevant because it takes into account the current climatic changes and the drought-related hydric stress (3). Also, because microbial communities represent huge complex systems, a global approach is necessary to understand the formation of consortia, communication between members, and functional interaction in a dynamic setting (6–9).
In recent years, there has been much progress in studying the metabolomics of several species of microorganisms, essentially taking advantage of using advanced equipment and highly efficient extraction techniques. To extract as much information as possible, a workflow should be defined to construct a microbial metabolome signature, as shown in Figure 1. A microbial metabolomics pipeline includes the sample preparation and the instrumental analysis steps, and a wide set of techniques that may be used to map the metabolome as completely as possible. For targeted and untargeted analysis of small volatile metabolites, gas chromatography–mass spectrometry (GC–MS) has been the most popular technique. However, comprehensive two-dimensional gas chromatography (GCxGC) has emerged as a technique with high potential for constructing a more detailed picture of the microbial metabolome (1–5,7,10).
FIGURE 1: Schematics showing a workflow that may be used for the construction of a microbial metabolomics platform, including the sample preparation step and the instrumental analysis component, in which gas chromatography is highlighted for targeted and untargeted analysis of volatile metabolites. Also, data processing and interpretation should be considered to have a complete metabolomics pipeline.
GC×GC advantages are based on the principle of the orthogonality mechanism that separates the constituents of the sample within a single analysis based on using two GC columns coated with different stationary phases. The interface samples small portions of the first dimension (1D) eluate, in general, by cryofocusing, and re-injects them into the second column (2D). Each 1D peak is modulated several times, largely preserving the 1D separation (11). Thus, compounds that are co-eluted from 1D undergo additional separation on 2D, and sensitivity and limits of detection are improved because they focus on the peak in the modulator and separate analytes from their chemical background. Also, the combination of the GC×GC instrument and a mass spectrometer with time-of-flight (ToF) analyzer allows the detection and quantification of analytes in picograms (pg), which is in line with the microbial metabolomics sensitivity requirements. The narrow peaks produced by GC×GC (peak width at half height of 0.1 s or less) require a detector with high data acquisition speed (hundred full-mass-range spectra per second), such as ToF-MS, thus providing sufficient data density. Moreover, ToF-MS allows the acquisition of full mass spectra at trace levels and mass spectral continuity, providing a reliable spectra deconvolution of overlapping peaks (10,11).
In constructing the microbial metabolome workflow, there are still several challenges to overcome, namely in the sample preparation stage. These challenges are related to a) microbial culturing in representative conditions; b) metabolic quenching approaches since strict control of the quenching procedure that arrests the cellular metabolism and enzymatic reactions should be done to reduce data variability; c) cell lysis approaches, depending on if the study is focused on the extracellular or intracellular metabolites, or both; and d) the extraction methodology that allows the determination of metabolites in representative conditions, and avoids the formation of artifacts or damage of metabolites since the amount of metabolites is very small. These challenges, alongside the technical difficulties to identify and quantify trace metabolites within complex matrices and the inherent problems related to data processing, are partially responsible for the paucity of information on the full volatile metabolome of common microbial pathogens or species with important biotechnological applications.
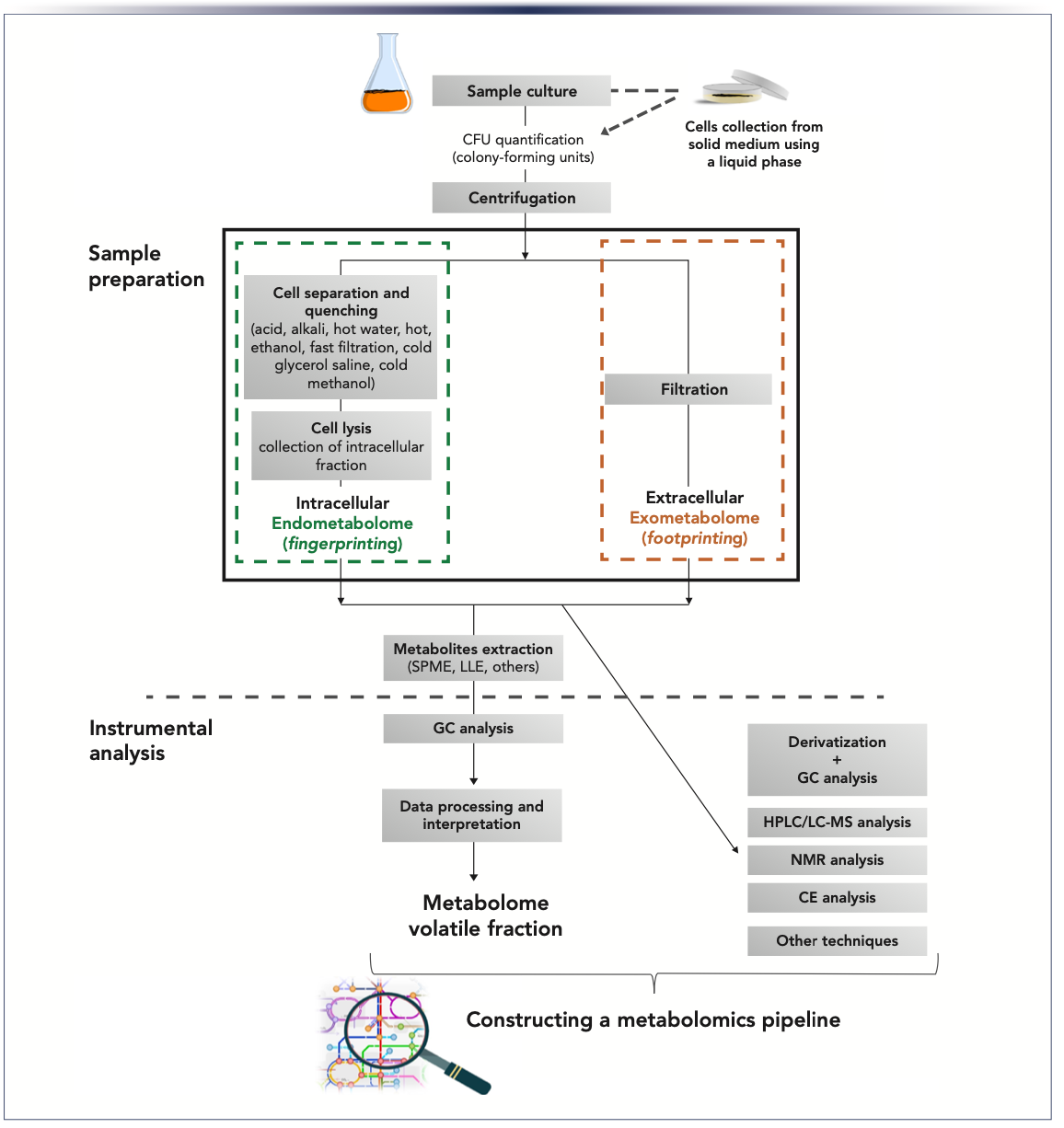
The definition of conditions for the culture of microorganisms is one of the first steps considered, where the selection of the medium is particularly important. Despite providing the appropriate nutrients into the culture medium, it is also necessary to attend the biophysical requirements during microorganism growth, such as oxygen levels, temperature, and the growth time. On the other hand, the volatile composition of the media must be minimal to avoid overlapping and interference with the metabolites produced by the microorganisms. Figure 2 illustrates the GC×GC total ion chromatogram contour plots of three culture media commonly used, which clearly shows the differences between the media regarding their volatile profiles, which is related to the composition of each medium as well as compounds that can be formed in the initial stage of sterilization. The volatile organic compounds (VOCs) screening medium exhibits a higher complexity of volatile composition compared with the others, which may be explained by the presence of the yeast extract (1% m/v). Thus, to confirm the origin of all detected analytes from microorganism cultures, excluding the medium effect, it is recommended to determine the amount of the analytes in the cultures and respective medium used as background control, analyzing both under the same conditions. To see if the VOCs do not exhibit significant differences between medium used as background control and microbes’ cultures, statistical significance should be verified by variance analysis. In general, the analytes not detected in the control medium or determined in the medium in levels at least three standard deviations lower than in the microbe’s culture for similar conditions, may be attributed as having their origin associated with the microbe’s metabolism (10). For instance, S. aureus strains were cultured in nonbuffered brain heart infusion (BHI) broth, and the analysis of variance tests indicated that 75 VOCs (most of them belonging to the chemical families of pyrroles, pyridines, furan-like compounds, pyrazines, Strecker aldehydes, and thiazoles) should not be considered as a microbe’s metabolites, and most of them are considered likely Maillard reaction products (1).
FIGURE 2: GC×GC total ion chromatogram contour plots of three culture media: (a) synthetic defined (SD) medium is based upon a yeast nitrogen base without amino acids supplemented with amino acids 0.67% m/v and glucose 2% m/v; (b) synthetic complete (SC) medium is based upon a yeast nitrogen base supplemented by a mixture of amino acids and vitamins; and (c) volatile organic compound (VOC) screening medium (yeast extract 1% m/v, diammonium phosphate ((NH4)2HPO4) 1% m/v, and glucose 2% m/v).
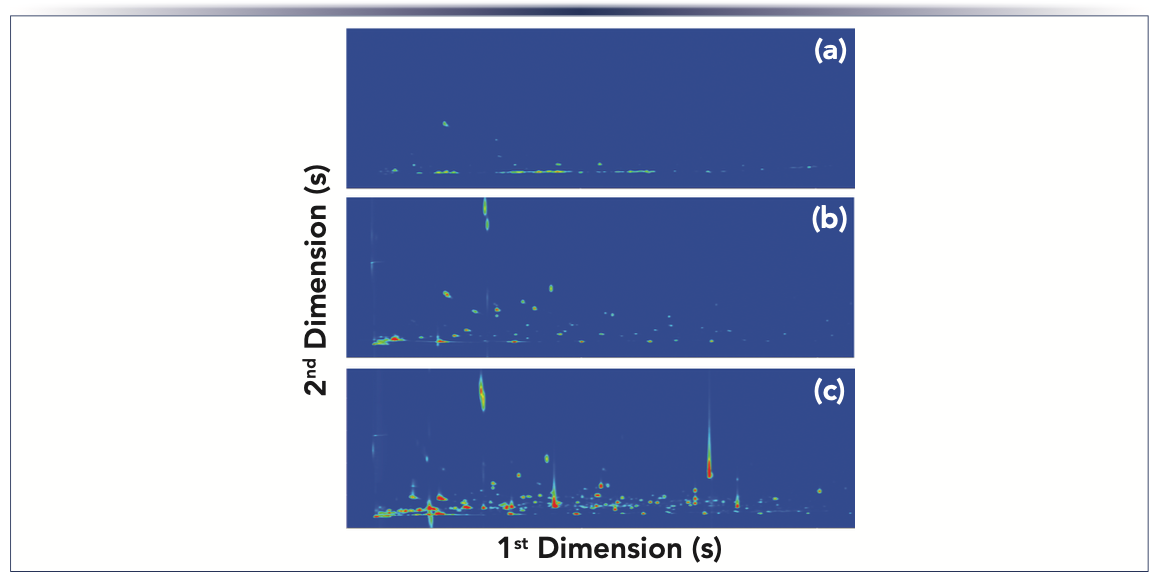
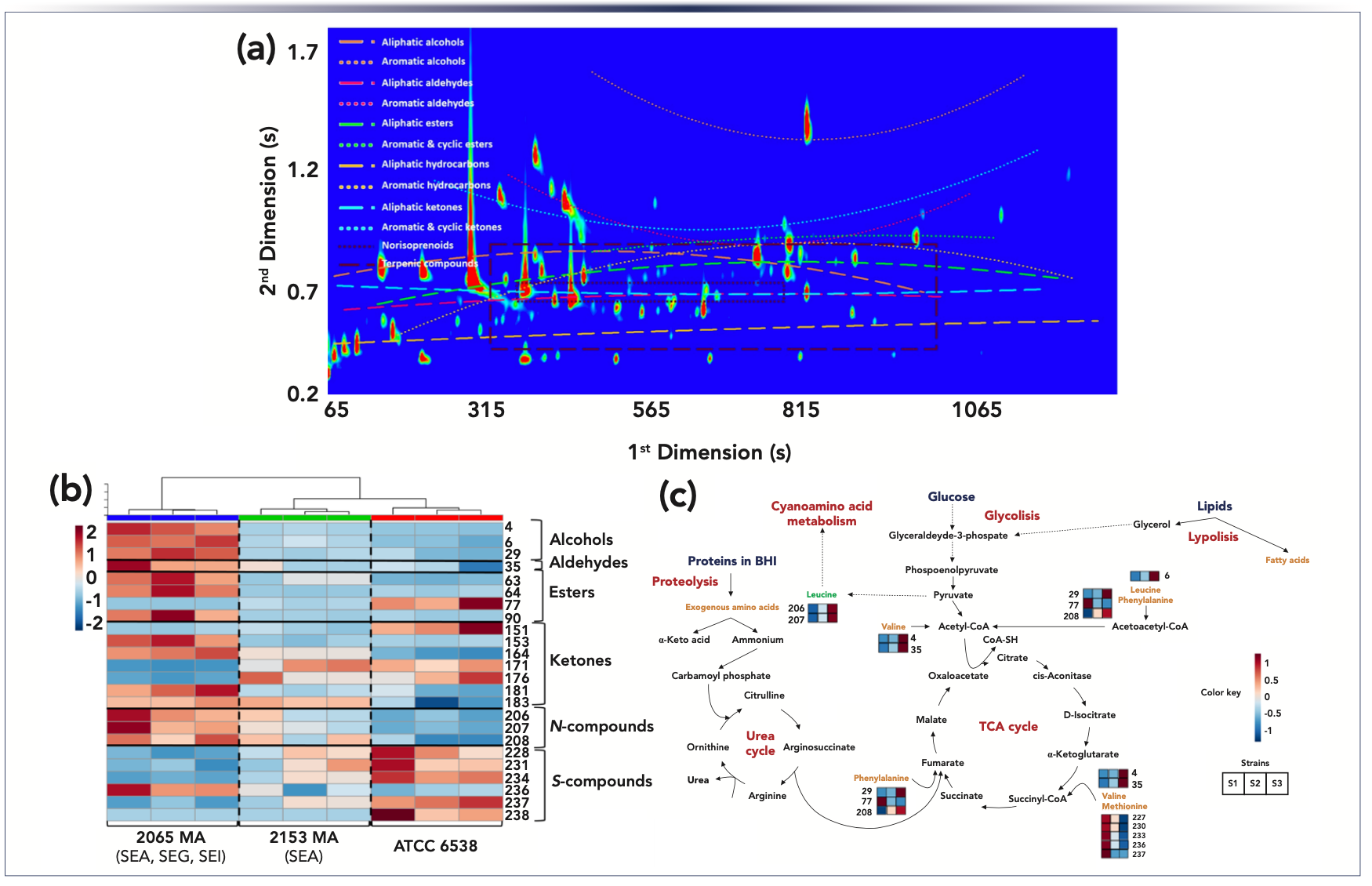
The methodology based on headspace solid-phase microextraction (HS-SPME)–GC×GC–ToF-MS provides novel data on the S. aureus volatile exometabolome (Figures 3a and 3b), showing higher complexity (240 volatiles) than previously reported (1). For untargeted profiling, the divinylbenzene/carboxen/poly(dimethylsiloxane) (DVB/CAR/PDMS) sorbent coating was selected as it is recommended for the extraction of a wide range of chemical species. This sorbent coating is produced using three different polymers, which gives it a synergistic effect between adsorption and absorption. This mutually synergetic effect promotes a higher retention capacity and, consequently, a higher sensitivity, which is very convenient to in-depth analyze complex samples such as the metabolites released by microorganisms. The metabolites released from S. aureus strains were mainly by-products of branched-chain amino acids and methionine degradation, pyruvate metabolism, diacetyl pathway, oxidative stress, and carotenoid cleavage (Figure 3c). The HS-SPME/GC×GC-ToFMS tandem with clustering analysis unveiled that the metabolites released by the first two pathways were produced in higher contents by the enterotoxic strains, which distinguished strains of S. aureus by the number of produced enterotoxins (1). This information is especially important from the food safety point of view.
FIGURE 3: (a) GC×GC–ToF-MS total ion chromatogram contour plot of the Staphylococcus aureus ATCC 6538 culture headspace volatile components. Volatiles chemical families used for statistical analysis are represented by the lines and clusters. The increase in volatility (low 1tR) is related to the decrease in the number of carbons through the first dimension. On the other hand, an increase in the 2tR correlates to an increase in polarity through the second dimension. (b) Hierarchical clustered heatmap visualization of the volatiles with variable importance in projection (VIP) values higher than 1.5 from the three strains of Staphylococcus aureus cultures headspace volatiles and organized by chemical families. The chromatographic area of each metabolite was normalized by CFU/mL followed by autoscaling. Each line corresponds to one metabolite and each column corresponds to each independent assay. (c) Metabolic pathways related with the VIP volatiles and their relative content in three strains of Stapylococcus aureus under study: S1 - ATCC 6538; S2 - 2153 MA; and S3 - 2065 MA. The relative content of the metabolite is illustrated on a red (high) to blue (low) chromatic scale. Adapted from reference (1).
Concluding Remarks
Despite the enormous interest in knowing the metabolome of microorganisms, information about subject, even for the most common species, is still scarce or unknown. The advances observed in recent years have essentially taken advantage of using sophisticated equipment such as GC×GC–ToF-MS instrumentation. Sample preparation is a multistep phase that continues to present many challenges, but the use of SPME is a more environmentally friendly alternative because it does not use solvents and is a simple and user-friendly technique. In addition, SPME allows the mapping of metabolites present in headspace, which have a central role in several biological phenomena of communication between species of microorganisms and microorganism-plants. The challenges related to data processing and interpretation, especially the difficulty to implement a computerized combined analysis of instrumental signals and metabolic pathway data bases (when available), are also noteworthy. Currently, methodologies for in situ analysis, such as for human biofluids, food, or numerous biotechnology applications, represent huge challenges in the field of microbial metabolomics. In these cases, the simultaneous presence of several species of microorganisms raises additional questions that can only be overcome with the use of equipment and sample preparation strategies of high efficiency, sensitivity, or specificity, as those previously reported.
Acknowledgments
Special thanks goes to the FCT/MEC for their financial support of LAQV-REQUIMTE (UIDB/50006/2020) Research Unit through national funds and where applicable co-financed by the FEDER, within the PT2020 Partnership Agreement.
References
(1) I. Baptista, M. Santos, A. Rudnitskaya, J.A. Saraiva, A. Almeida, and S.M. Rocha, Int. J. Biochem. Cell Biol. 108, 40–50 (2019). doi:10.1016/j.biocel.2019.01.007
(2) P. Cardoso, M. Santos, R. Freitas, S.M. Rocha, and E. Figueira, Environ. Pollut. 231, 802–811 (2017). doi: 10.1016/j.envpol.2017.08.067
(3) D.R. Matos, C.F. Sá, P.J. Cardoso, A. Pires, S.M. Rocha, and E. Figueira, Ecotoxicol. Environ. Saf. 186, 109759 (2019). doi:10.1016/j.ecoenv.2019.109759
(4) Z. Alves, A. Melo, A.R. Figueiredo, M.A. Coimbra, A.C. Gomes, and S.M. Rocha, PLoS One 10(11), 1–16 (2015). doi: 10.1371/journal.pone.0143641
(5) C. Martins, T. Brandão, A. Almeida, and S.M. Rocha, J. Sep. Sci. 40, 2228–2237 (2017). doi:10.1002/jssc.201601296.
(6) J. Tang, Microb. Genom. 12, 391–403 (2011). doi: 10.2174/138920211797248619
(7) C.P. Costa, A.R. Bezerra, A. Almeida, and S.M. Rocha, Microorganisms 8, 1911 (2020). DOI:10.3390/microorganisms8121911.
(8) D.S. Childers and D. Kadosh, PLoS One 10, 1–15 (2015). doi: 10.1007/978-1-4939-8757-3_2
(9) M. Martins, M. Henriques, J. Azeredo, S.M. Rocha, M.A. Coimbra, and R. Oliveira, J. Basic Microbiol. 50, 1–9 (2010). doi: 10.1002/jobm.200900442
(10) C.P. Costa, D.G. Silva, A. Rudnitskaya, A. Almeida, and S.M. Rocha, Sci. Rep. 6(27441), 1–13 (2016). doi:10.1038/srep27441.
(11) S.E. Prebihalo, K.L. Berrier, C.E. Freye, H.D. Bahaghighat, N.R. Moore, D.K. Pinkerton, and R.E. Synovec, Anal. Chem. 90, 505–532 (2018). doi.org/10.1021/acs. analchem.7b04226
Sílvia M. Rocha is with the Chemistry Department and LAQV/REQUIMTE at the University of Aveiro, in Aveiro, Portugal. Direct correspondence to: smrocha@ua.pt.

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.














