Evaluation of the Gas-Liquid Separator in the Mass-Directed SFC Prep System with Open-Bed Collection
With many "blockbuster" drugs losing patent protection, there has been mounting pressure across the pharmaceutical industry to shorten the time to bring new chemical entities (NCEs) to market and reduce the overall cost. This requires concerted productivity improvement in many aspects of drug discovery and development by continuously adopting new enabling technologies.
Lakshmi Subbarao, Daniel Rolle, and Rui Chen, Waters Corporation
With many "blockbuster" drugs losing patent protection, there has been mounting pressure across the pharmaceutical industry to shorten the time to bring new chemical entities (NCEs) to market and reduce the overall cost. This requires concerted productivity improvement in many aspects of drug discovery and development by continuously adopting new enabling technologies. Recently, there has been great interest in implementing a mass-directed preparative supercritical fluid chromatography (SFC) system with an open-bed collection format for high throughput purification due to the many intrinsic advantages SFC can offer including high efficiency chromatography, unique selectivity, and nonaqueous purification resulting in shorter fraction dry-down times (1–4).
In preparative SFC, fractions are collected downstream of a back pressure regulator (BPR). Upon exiting the BPR, liquid CO2 in the mobile phase undergoes rapid expansion, causing extensive spraying and aerosol formation. Without proper control, this leads to sample loss and/or cross contamination. This is typically addressed by a gas-liquid separator (GLS) prior to collection. For batch purification with open-bed collection, where each injection contains a unique sample and effluent flows through the GLS continuously to the collection bed, removing CO2 with the GLS and flushing between closely eluting peaks in a run as well as between injections has to be fast yet highly efficient to minimize cross contamination inter- and intra-injections.
Previous generation mass-directed SFC purification systems with open-bed collection circumvented the aforementioned issues by using flow rates less than 30 g/min and positioning the collection probe deep into a large collection tube (5–6). While these systems have proven moderately successful, the low flow rates are perceived by many as impractical in pharmaceutical high throughput purification environments.
In early 2009, Waters Corporation launched the Waters SFC-MS Prep 100 system using a new proprietary GLS (Figure 1), with flow rates up to 100 g/min enabling purifications of ~100 mg/injection. Herein, we describe the working principles of the new GLS and demonstrate its functionality through a set of simple experiments.

Figure 1
Experimental
All experimentation was performed using an SFC-MS Prep 100, which consists of a Waters 2767 sample manager, Waters 3100 MS detector, Waters 2998 photodiode array (PDA) detector, TharSFC CO2 pump and cosolvent pump, Waters 515 pumps, TharSFC column oven, TharSFC automated back pressure regulator (ABPR), TharSFC GLS, and TharSFC tunable splitter. The system is controlled by Waters MassLynx™ software.
A Viridis™ 2-ethylpyridine column (19 × 150 mm, Waters Corporation, Milford, MA) was used. A stock solution of carbamazepine and ketoprofen (25 mg/mL each) was prepared in methanol. The chromatographic conditions were: flow rate: 100 g/min; cosolvent: methanol; cosolvent percentage: 15%; column oven temperature: 40 °C; system pressure: 120 bar. Five replicate 2 mL injections of the stock solution (100 mg total loading) were made and each component was collected, with a 5 s rinse after each collection. Each fraction was then diluted to 50 mL. A standard solution was prepared by diluting 2 mL of the stock solution to 50 mL. The diluted fractions and standard solution were analyzed on a Viridis™ 2-ethylpyridine column (4.6 × 150 mm, Waters Corporation) using an analytical SFC-MS Resolution system. All chromatographic conditions were kept the same, with the exception of flow rate and injection volume which were adjusted to 5 mL/min and 5 μL, respectively. The peak area ratios of the diluted collections and the standard solution were calculated as recovery and purity was estimated by relative peak area.
Results and Discussion
Figure 1 is the schematic of the proprietary GLS. In short, the geometry of the GLS, the dimension of the intruding tube or "initiator," and its angle with respect to the inner wall concertedly induce the effluent in a cyclonic flow path towards the bottom of the GLS while gaseous CO2 escapes from the vent on top of the GLS. The GLS is pressurized to ~ 40 psi to ensure CO2 expansion in a controlled manner. This positive pressure also coerces liquid flow towards the bottom of the GLS. To compensate for the variation in the time required for effluent to pass through the GLS, a make-up liquid pump is used to deliver methanol to the GLS using a predefined algorithm, such that the time delay between UV-MS signal and collector tip remains relatively constant (3).

Figure 2
Figure 2 displays the MS total ion chromatogram (TIC) and PDA total absorbance chromatogram of the carbamazepine/ ketoprofen mixture. Note that the chromatography was purposely adjusted to generate a pseudo "touching-band" separation where only a 5 s rinse was performed to gauge the true effectiveness of GLS (7). Figure 3 shows the re-analyses of the fractions with respect to the standard solution and the results are listed in Table I. Not surprising, the purity of carbamazepine was 100%; whereas the purity of ketoprofen was estimated to be 99.4%. The average recoveries were 98.7% for carbamazepine and 91.2% for ketoprofen. These results indicate that even under the stringent condition, a 5 s rinse sufficed to minimize carryover between neighboring peaks. This is in agreement with the independent evaluation by Aurigemma, et. al. (2). Clearly, the GLS was effective in removing CO2 and minimizing aerosol formation, leading to high recovery and purity. In addition, the make-up methanol delivered to the GLS also plays a key role, especially when cosolvent percentage is low (<20%). The contributions of the make-up flow are two-fold. First, the combined liquid flow from the mobile phase and the make-up pump enables effluent travel through the GLS with a relative high velocity, hence, maintaining peak integrity as well as a constant time delay between UV-MS signal and collector for correct collection triggering to ensure high recovery. Second, the combined liquid flow allows sufficient sweeping of the flow path to minimize residual peak carryover into the subsequent collection, in as short as 5 s, for high purity collection.
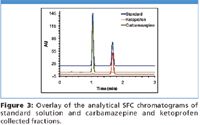
Figure 3
Conclusions
Closely eluting carbamazepine and ketoprofen were collected using the SFC-MS Prep 100 and the resulting fractions were re-analyzed using an analytical SFC-MS. Purity and average recovery were 100% and 98.7% for carbamazepine and 99.4% (estimated) and 91.2% for ketoprofen, respectively. The results indicate the effectiveness of the GLS for high purity and high recovery collection, with a 5 s rinse between neighboring peaks. In addition to the design of the proprietary GLS, make-up flow also contributes significantly to the overall success. The new GLS has overcome the main historical hurdle for implementing SFC collection in an open-bed format with flow rates up to 100 g/min.
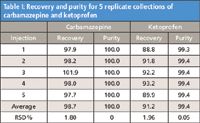
Table I: Recovery and purity for 5 replicate collections of carbamazepine and ketoprofen
References
(1) R.T. McClain, A. Dudkina, J. Barrow, G. Hartman, and C.J. Welch, J. Liquid Chromatog. & Related Tech., 32, 483–499 (2009).
(2) C. Aurigemma, S. Zulli, Z. Wang, and J.L. Lefler, poster presentation, LabAutomation, Palm Springs, CA, USA (2009).
(3) R. Chen, LCGC Europe EApplication Note Alert, Feb. 2010.
(4) A. Mich, B. Matthes, R. Chen, and S. Buehler, LCGC Europe: The Application Notebook, 12–13, March 2010.
(5) T. Wang, M. Barber, I. Hardt, and D.B. Kassel, Rapid Commun. Mass Spectrom., 15, 2067–2075, (2001).
(6) X. Zhang, M. H. Towle, C.E. Felice, J.H. Flament, and W. K. Goetzinger, J. Comb. Chem., 8, 705–714 (2006).
(7) L.R. Snyder, J.J. Kirkland, and J.L. Glajch, "Practical HPLC method development", John Wiley & Sons, Inc., 2nd Ed., 616–618 (1997).

Waters Corporation
575 Epsilon Drive, Suite 100, Pittsburgh, PA 15238
tel. (412)967-5665; fax (412)967-9446
Email: info@tharsfc.com; Website: www.waters.com/sfc

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.














