The Essentials: Optimizing Sample Introduction for Headspace GC
The Essentials: Optimizing Sample Introduction for Headspace GC
An excerpt from LCGC’s e-learning tutorial on headspace gas chromatography (GC) at CHROMacademy.com

(Photo Credit: Jose Luis Pelaez/Getty Images)
Static headspace sampling is typically used for the determination of volatile and semivolatile analytes in liquids and, more rarely, solid matrices. Application examples include the analysis of alcohols in blood, residual solvents in pharmaceuticals, flavours and taints in food and beverages, and fragrances in perfumes and detergents. Samples are heated and agitated at a set temperature for a set time, after which an aliquot of the headspace gas is analyzed by gas chromatography (GC) to determine the concentration of the analyte of interest in the headspace, which can then be related to the concentration in the original sample. The vapour pressure of a compound above a solution is directly proportional â¨to its mole fraction in that solution multiplied by an activity coefficient. The activity coefficient relates to the degree â¨of intermolecular attraction between â¨the analyte and the other species within the sample. The following equations are most often used to describe the basis of headspace determination:
C
G
=
C
O
/(
K
+
V
G
/
V
L
) [1] where
C
G
is the analyte concentration in the gas phase,
C
O
is the analyte concentration in the original sample,
K
is the partition coefficient (2),
V
G
is the volume of headspace gas, and
V
L
is the sample volume.
K
=
C
S
/C
G
[2] where
C
S
is the analyte concentration in the sample liquid and C
G
is the analyte concentration in the headspace gas. To determine
K
it is necessary to calibrate instrument response by analyzing standards containing a known amount of analyte. It is very important that the standard is matrix matched to the analyte because the matrix components can significantly affect the activity coefficient of the analyte as described above. When determining ethanol in water a
K
value of around 500 is not unusual, indicating that there is around 500 times more ethanol in the water at equilibrium than in the headspace, again not unusual given the high solubility of ethanol in water because of the comprehensive hydrogen bonding between the analyte and matrix. When determining hexane in water,
K
values of 0.01 are not unusual, meaning there is 100 times (1/0.01) more hexane in the headspace. How would these figures be affected by changing the various experimental variables?
Sample Volume
Increasing sample volume will not significantly affect the headspace concentration for analytes with high values of
K
. For intermediate values of
K
(~10), the increase in sample volume is approximately linear and for analytes with low values of
K
an increase in sample volume will give a large proportional increase in headspace concentration.
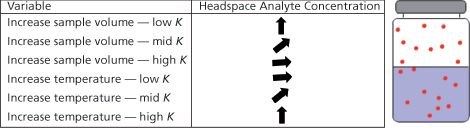
Figure 1: Headspace analyte concentration in a sample vial as a function of sample volume and temperature. Low analyte headspace concentrations caused by good analyte solubility in the matrix cannot be significantly improved by increasing sample volume. Use around 10 mL of sample (if available) in a 20-mL headspace vial. This also makes the phase ratio (β =
V
G
/
V
L
) equal to 1 and simplifies calculations.
Temperature
Samples with a high value of
K
will be significantly affected by temperature, and increasing temperature is a good way to improve headspace concentration. However, to obtain good precision, one needs to carefully and accurately control the equilibration temperature and for analytes with
K
values of 500, a temperature accuracy of ±0.1 °C is required to obtain a precision of 5%! With analytes where
K
is low, increasing the temperature has a lesser effect and can even cause a reduction in analyte headspace concentration. One special note here is that as temperature is increased when using aqueous samples, the overall headspace pressure can increase markedly and the sudden release of pressure on inserting the sampling needle may case loss of analyte or a significant dilution effect.
Equilibration Time
Headspace equilibration time will depend on analyte vapour pressure, concentration in the sample, phase ratio, and temperature or agitation. Do not be tempted to draw a correlation between equilibration time and partition coefficient value. Each analyte or sample combination and sample to headspace ratio will need to be investigated to determine the time required to reach equilibrium for each analyte.
Salting Out
The partition coefficient of polar analytes in polar matrices can be significantly reduced by adding a very high concentration of salt (potassium chloride is typical) to the sample matrix.
Instrument Variables
When using autosampler devices, use the smallest volume sample loop that gives the required signal-to-noise ratio. The sample, loop, transfer line, and inlet temperatures should be offset by at least +20 °C to avoid sample condensation. If the signalâto-noise ratio allows, applying a small split flow of 10:1 often improves analyte peak shape and makes peak area measurement more reproducible.

Study Examines Impact of Zwitterionic Liquid Structures on Volatile Carboxylic Acid Separation in GC
March 28th 2025Iowa State University researchers evaluated imidazolium-based ZILs with sulfonate and triflimide anions to understand the influence of ZILs’ chemical structures on polar analyte separation.












