Authentication and Routine Screening of Ginsenoside Isomers in Functional Food Products: UHPLC Coupled with Ion Mobility Mass Spectrometry
Ginseng has been used worldwide for thousands of years. Thought to possess therapeutic effects, it has been marketed as a natural product for the treatment of disease. This article describes how ultrahigh-performance liquid chromatography (UHPLC) can be coupled with ion mobility mass spectrometry (IMS-MS) to profile phytochemicals contained within ginseng and confirm quality.
Ginseng has been used worldwide for thousands of years. Thought to possess therapeutic effects, it has been marketed as a natural product for the treatment of disease. This article describes how ultrahigh-performance liquid chromatography (UHPLC) can be coupled with ion mobility mass spectrometry (IMS-MS) to profile phytochemicals contained within ginseng and confirm quality.
(Photo Credit: TongRo Images/Getty Images)

The popularity of nutraceutical and functional food products - found in foods, roots, and herbs - continues to increase globally bringing about the introduction of new legislation for analyzing active compounds in natural products. Such legislation was brought into effect on 30 April 2011 and resulted in the ban of hundreds of traditional herbal remedies in Europe under the Directive 2004/24/EC.1 Regulations have recently been restricted further, allowing only well-established and quality-controlled medicines to be sold, as well as products assessed by the Medicine and Healthcare Products Regulatory Agency (MHRA). Manufacturers now have to prove that their products have been made to strict standards and contain a consistent and clearly marked dose.
The roots of ginseng plants have been used for medicinal purposes for thousands of years and there are a number of different species that are thought to possess different therapeutic properties - including CNS stimulant activity, hypoglycemic properties, and sedative effects.2 Korean ginseng is one of the most widely used herbs in the world. Its scientific name is Panax ginseng, which is the species from which Chinese, Korean, red, and white ginseng are produced. Chinese and Korean ginseng are the same plant cultivated in different regions, and have slightly different properties according to Chinese medicine. White ginseng is simply the dried or powdered root of Korean ginseng, while red ginseng is the same root that is steamed and dried in heat or sunlight. Red ginseng is said to be slightly stronger and more stimulating in the body than white, according to Chinese herbalism.
Ginsenosides are part of a diverse group of steroidal saponins with a four ring structure similar to steroids that can be classified into two main groups: the panaxadiol (Rb1 group) that includes Rb1, Rb2, Rc, Rd, Rg3, Rh2, and Rh3; and the panaxatriol (Rg1 group) that includes Rg1, Re, Rf, Rg2, and Rh1. American ginseng is richer in the Rb1 group of ginsenosides whereas Korean ginseng is richer in the Rg1 group.3 The phytochemical profile of the two species can also be affected by the time of harvest, storage conditions, and production processes. Manufacturers are therefore required to determine the quality and potency of ginseng products in line with regulations, to ensure the phytochemical content is as labelled.
This article demonstrates how ultrahighâperformance liquid chromatography (UHPLC) coupled with ionâmobility mass spectrometry (IMSâMS) can be an ideal method for profiling complex mixtures from natural products. IMS-MS is a rapid orthogonal gas phase separation technique that allows another dimension of separation to be obtained within an LC timeframe and differentiates compounds based on size, shape, and charge. A screening assay to explore the use of UHPLC separations with ion mobility mass spectrometry for the characterization of the distribution and content of mono-, di-, and tetra-glycosides in raw material or processed products is presented and illustrated. In this case, the aim is to illustrate how the quality and potency of ginseng products can be determined.
Screening and Confirming Isomer Markers with Collision Cross-Section Values
A collision cross-section (CCS) value is a robust, measurable physicochemical property of an ion that is an important distinguishing characteristic related to its chemical structure and threeâdimensional conformation. This article presents TWCCSN2 values (derived from ion mobility drift times) as a new identification parameter that can distinguish ginsenoside isomers as well as unknowns.4Method: Non-targeted UHPLC–IMS-MS was used to generate travelling wave collision cross-sections using a nitrogen buffer gas (TWCCSN2), accurate mass precursor/fragment ions, and retention times to profile ginsenoside standards Rb1, (Rb2, Rc), (Rd, Re), (Rf, Rg1), and Rg2 (100 pg/μL). The CCS measurements generated were then entered into a scientific library within the software UNIFI (Waters), allowing the expected and determined TWCCSN2 values to be used to screen and confirm the presence of ginsenoside isomer markers. Korean ginseng tea (extract in 20 mL of H2O), gingko biloba, and red panax (undiluted) extracts were analyzed. These were screened against the created ginsenoside TWCCSN2 library in UNIFI to determine the presence/unequivocal identification of ginsenoside isomers. A Waters Acquity UPLC I-Class System and Synapt G2-Si Mass Spectrometer was used in the analysis.
Figure 1: UHPLC–IMS-MS electrospray negative mode conventional base peak ion chromatogram obtained for analysis of undiluted Korean ginseng tea extract.
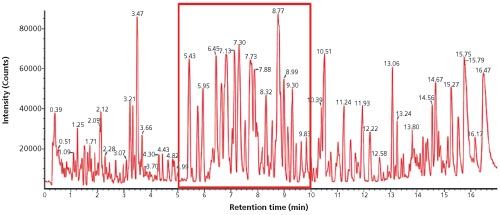
Results: Figure 1 shows the UHPLC–IMS-MS electrospray negative mode conventional base peak ion chromatogram obtained for the analysis of undiluted Korean ginseng extract to show the complexity of the sample profiled. Figure 2 presents the UHPLC–IMSâMS electrospray negative mode plot of drift time (ion mobility resolution) versus retention time for the Korean ginseng tea extract and illustrates how an ion mobility separation, orthogonal to a chromatographic separation, can increase peak capacity.
Figure 2: UHPLC–IMS-MS electrospray negative mode plot of drift time (ion mobility resolution) versus retention time for Korean ginseng tea extract.
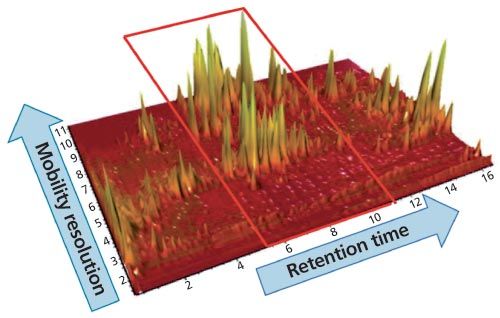
The retention time region between 6 min and 10 min shows that there are a large number of compounds that are now resolved compared to the same region on the conventional base peak ion extracted mass chromatogram of Figure 1. The true complexity of the sample profiled is illustrated when ion mobility resolution and UHPLC chromatographic resolution are combined.
Resolving Co-Eluting Compounds with Ion Mobility: Figure 3 shows that the combined peak capacity of UHPLC and ion mobility can have other advantages in addition to serving as an additional identification point. The retention time (7.73 min) and drift time (9.93 ms), aligned precursor, and product ion spectra for Rc ginsenoside marker isomer is shown. The spectra presented only result from the Rc ginsenoside because chromatographically co-eluting compounds are resolved using ion mobility. The ion mobility spectral cleanup makes it clear that the unknown isomer at 7.88 min has the same characteristic fragment ions as ginsenoside Rc, but it can be differentiated using ion mobility. It is also worth noting that these acquisitions are non-targeted; hence the TWCCSN2 values are generated for all knowns and unknowns. The characteristic information acquired and processed for an unknown isomer is shown in Figure 4. The candidate component summary, mobility trace, and precursor/mobility product ion spectra for the unknown isomer with TWCCSN2 = 358.80Å2 at retention time 7.88 min are illustrated. The TWCCSN2 value for the unknown isomer can be entered into the scientific library, along with the TWCCSN2 values generated for all of the compounds detected during the chromatographic run.
Figure 3: Retention time (7.73 min)/drift time aligned (9.93 ms) precursor and ion mobility product ion spectra for Rc ginsenoside marker isomers, where their characteristic fragmentation pattern and proposed fragmentation pathways are shown. (Click on image to enlarge).
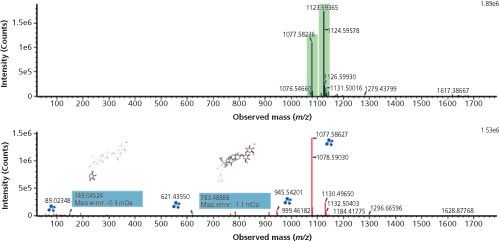
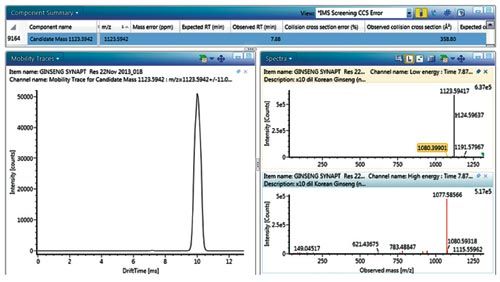
Figure 4: UNIFI Component Summary illustrates the candidate component summary, mobility trace, and precursor/mobility product ion spectra for an unknown isomer TWCCSN2 = 358.80Å2 at retention time 7.88 min.
Identification with Collision Cross-Section Measurements: CCS measurements can increase confidence in identification as demonstrated by the results obtained from profiling Korean ginseng over two consecutive weeks. For the marker ginsenoside isomer pairs (Rb2, Rc), TWCCSN2 measurements of 361.77 Å2/350.58Å2 have been determined. For Rd, Re, 328.89 Å2/333.11 Å2 were determined. For Rf, Rg1, 304.7 Å2/295.83 Å2 were obtained in week one. In week two, comparative results were obtained. When comparing the expected against the measured TWCCSN2 results determined (for the eight ginsenosides profiled in the extracts), the measurement errors were typically <0.5%. It is therefore possible to distinguish the marker isomer pairs of ginsenosides in the extracts of the specified products analyzed with confidence using TWCCSN2 measurements.
Summary
In conclusion, the results clearly show the benefits of using CCS measurements and the combined peak capacity of UHPLC with IMSâMS. Coeluting analytes and isomers were resolved and identified in the three extracts profiled. In addition it was possible to acquire the cleaned up mobility specific product ion spectra, which are ion mobility resolved from coeluting components. In this case the reason for such a screening approach is to generate a new analysis to enable the characterization and content of mono-, di-, and tetra-glycosides in the raw material or processed functional food or nutraceutical products. This assay approach could add confidence when assessing quality, potency, and consistency of a final product, incorporating ingredients such as gingko biloba, Korean ginseng, and red panax.
While also gaining confidence in the identifications made from the use of accurate mass measurement and CCS measurements, there is also the clear potential to reduce the amount of high purity standards required, where confirmation relies on retention time and accurate mass measurement. The use of a CCS screening approach has the potential to provide significant cost savings across many application areas. In further studies to profile ginsenosides, Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, and Rg2, the consumption and costs of high purity standards has been significantly reduced within the laboratory where the study was performed.5
References
- DIRECTIVE 2004/24/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 31 March 2004 http://ec.europa.eu/health/files/eudralex/vol-1/dir_2004_24/dir_2004_24_en.pdf
- T. Ligor, A. Ludwiczuk, T. Wolski, and B. Buszewski, Anal. Bioanal. Chem.383(7–8), 1098–105 (2005).
- A.S. Attele, J.A. Wu, and C.S. Yuan, Biochem. Pharmacol. 58, 1685–1693 (1999).
- S.D. Pringle, K. Giles, J.L. Wildgoose, J.P. Williams, S.E. Slade, K. Thalassinos, R.H. Bateman, M.T. Bowers, and J.H. Scrivens, International Journal of Mass Spectrometry261, 1–12 (2007).
- M. McCullagh, R. Lewis, and D. Douce Investigating UPLC Ion Mobility Mass Spectrometry: A new Approach To Authentication and Routine Screening of Ginsenoside Isomers In Functional Food Products. Waters application note 720005422en
- The use of Collision Cross Section (CCS) Measurements in Food and Environmental Analysis. Waters Technical Note No. 720005374en, April 2015.
Michael McCullagh has worked for Waters Corporation since 2001, performing sample analysis in the food and environmental, pharmaceutical/life science and validation departments. Using time of flight technology, he has gained experience in a number of application areas, such as metabolite ID, impurity profiling, natural product profiling, authentication profiling, and pesticide/veterinary residue screening. His experiences in these application areas have been used to explore the utility of ion mobility mass spectrometry.
Rob Lewis has 21 years of experience in the MS industry, working on quadrupole and time-of-flight LC–MS systems. For the last 12 years he has been working in the MS systems evaluation department, which is responsible for ensuring the development of MS instruments meet user performance requirements. During this time he has worked on many application solutions including a natural product application solution where he gained experience working with compounds such as the ginsenosides.
David Douce obtained his Ph.D in 1998 from Sheffield Hallam University (UK) in the area of environmental monitoring using HPLC and GC–MS. After working for an environmental testing laboratory and a large CRO he moved to Micromass/Waters in 2000. He initially worked in the applications laboratory performing sample analysis on a wide range of application areas before moving into the evaluation group where all new mass spectrometric products (both quadrupole and time of flight-based) are tested before they are released. His current areas of interest include source technologies and the practical use of these in a diverse number of application areas.
E-mail: Mike_McCullagh@waters.com
Website: www.waters.com

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.








