Enhancing Phosphotyrosine Proteome Coverage Using a Combined ETD and CID Approach on a LTQ Orbitrap XL ETD
The Application Notebook
Collision-induced dissociation (CID) and electron transfer dissociation (ETD) are complementary mass spectrometric fragmentation techniques. We have used CID and ETD in different approaches to analyse tyrosine phosphorylation using a Thermo Scientific LTQ Orbitrap XL equipped with ETD.
S. Lemeer,1,2 M. Zeller,3 A.F.M. Altelaar,1,2 T. Moehring,3 S. Mohammed1,2 and A.J.R. Heck,1,2
1Biomolecular Mass Spectrometry and Proteomics Group, Bijvoet Center for Biomolecular Research and Utrecht Institute for Pharmaceutical Sciences, Utrecht University, Utrecht, The Netherlands,2Netherlands Proteomics Centre, Utrecht, The Netherlands,3Thermo Fisher Scientific, Bremen, Germany.
Collision-induced dissociation (CID) and electron transfer dissociation (ETD) are complementary mass spectrometric fragmentation techniques. We have used CID and ETD in different approaches to analyse tyrosine phosphorylation using a Thermo Scientific LTQ Orbitrap XL equipped with ETD.
Reversible tyrosine phosphorylation plays important roles in numerous cellular processes and is tightly controlled by the balanced action of protein-tyrosine kinases and protein-tyrosine phosphatases. In multiple cancers, aberrant tyrosine phosphorylation has been suggested to be the underlying cause (1). Therefore, the detection and site specific localization of tyrosine phosphorylation has emerged over the past years.
Low abundances and the dynamic nature of tyrosine phosphorylation together with the higher abundance of non-phosphorylated peptides cause detection of this modification to be problematic. A variety of strategies ranging from mass spectrometric (MS) based methods to phosphopeptide enrichment prior to MS have been developed (2–4). Immunoaffinity enrichment by antibodies directed against the tyrosine phosphorylated residue can be used for both tyrosine phosphorylated proteins and peptides (2,3,5).
Despite recent advances, the tyrosine phosphoproteome is far from comprehensive and, therefore, ongoing method development is still essential. Here, we used the LTQ Orbitrap XL ETD to identify the phosphorylation sites from a peptide immunoaffinity purification of pervanadate treated HeLa cells.
Materials and Methods
Nanoflow LC–MS/MS was performed by coupling an Agilent 1100 HPLC system (Agilent Technologies, Waldbronn, Germany) to a LTQ Orbitrap XL ETD mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) as described previously (6). The LTQ Orbitrap XL ETD performed a full MS scan (RP 60000 FWHM) followed by data-dependent CID and ETD MS/MS scans with detection of the fragment ions in the linear ion trap. The decision tree method was set up to fragment all doubly charged ions, all triply charged ions above m/z 650, all quadruply charged ions above m/z 900 and all higher charged ions above m/z 950 with CID and all other precursor ions with ETD.
Results
The analytical strategy is based on the use of the two complimentary dissociation techniques collision induced dissociation (CID) and electron transfer dissociation (ETD) for the identification of the peptide as well as for the determination of the tyrosine phosphorylation site. Two approaches are compared: Fragmentation of every peptide by both CID and ETD, and the use of the most efficient dissociation technique depending upon the peptide's property such as mass-to-charge ratio m/z and charge state z (so-called data-dependent decision tree, DDDT) (7).
With the first approach 154 unique tyrosine phosphorylated peptides (Mascot score ? 20) were identified with the majority of sites (56%) from both CID and ETD spectra Figure?1(a). However, 35 phosphopeptides were exclusively identified with CID, whereas 32 phosphopeptides were exclusively identified with ETD. In the DDDT experiment, a total number of 200 unique tyrosine phosphorylated peptides were identified. Only 13% of the peptides (26 phosphopeptides) were identified from both CID and ETD spectra Figure?1(b). 128 phosphopeptides were identified exclusively after CID fragmentation and 51 phosphopeptides were identified exclusively after ETD fragmentation. This resulted in a 30% increase in the total number of tyrosine phosphopeptide identifications in the DDDT method, clearly indicating that this method is superior to the classic method.
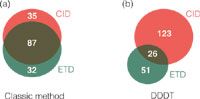
Figure 1: Identified tyrosine phosphorylated peptides from ‘classic’ and DDDT method. (a) Overlap between identified phosphopeptides identified from CID and ETD in the same run using the classic method, systematically performing CID and ETD fragmentation on the same peptide precursor. (b) Overlap between identified phosphopeptides identified from CID and ETD in the same run using the DDDT method, choosing the optimal dissociation method for each peptide precursor.
Conclusion
We have shown that CID and ETD are truly complementary fragmentation techniques and their combined use greatly enhances the phosphoproteome coverage compared to MS methods that are solely based on CID. We have shown that a significant number of phosphopeptides can only be identified by ETD and the intelligent use of CID and ETD maximizes the outcome of our phosphoproteomics experiment.
References
1. P. Blume-Jensen and T.Hunter, Nature, 411, 355–365 (2001).
2. J. Rush et al., Nat. Biotechnol., 23, 94–101 (2005).
3. Y. Zhang et al., Mol. Cell. Proteomics, 4, 1240–1250 (2005).
4. H. Steen et al., J. Biol. Chem., 277, 1031–1039 (2002).
5. P.J. Boersema et al., Mol. Cell. Proteomics, 9, 84–99 (2010).
6. M.W. Pinkse et al., J. Proteome Res., 7, 687–697 (2008).
7. D.L. Swaney, G.C. McAlister and J.J. Coon, Nat. Methods, 5, 959–964 (2008).

Thermo Fisher Scientific
Hanna-Kunath-Strasse 11, 28199 Bremen, Germany
Website: www.thermoscientific.com

A Guide to (U)HPLC Column Selection for Protein Analysis
April 16th 2025Analytical scientists are faced with the task of finding the right column from an almost unmanageable range of products. This paper focuses on columns that enable protein analysis under native conditions through size exclusion, hydrophobic interaction, and ion exchange chromatography. It will highlight the different column characteristics—pore size, particle size, base matrices, column dimensions, ligands—and which questions will help decide which columns to use.
The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.














