Electrostatic Repulsion Hydrophilic Interaction Chromatography
LCGC North America
ERLIC is a new technique that shows promise as an alternative or complementary technique for both enrichment and separation of these posttranslationally-modified peptides.
Electrostatic Repulsion Hydrophilic Interaction Chromatography — A New Tool for Enrichment of Phospho- and Glycopeptides
Introduction of modifications into the protein backbone or its amino acid side chains following the translation of the mRNA code enables a single polypeptide chain to be transformed into diverse species with enhanced or modified biological functions. These posttranslational modifications (PTMs) can affect the cellular location of a protein, its biological lifetime, and its function or activity level. Of the huge variety of PTMs observed in the proteome, phosphorylation and glycosylation are among the most important. Reversible phosphorylation at serine, threonine, and tyrosine residues is implicated in control of cell growth and differentiation, cell death, gene expression, and signal transduction. Glycosylation at serine, threonine, and asparagine residues modulates the structure and function of membrane and secreted proteins and is important in proper protein folding and subcellular localization. Given the importance of these two posttranslational modifications in cell metabolism, the characterization of phosphoprotein and glycoprotein structure and function is vital to the identification of drug targets and development of novel therapeutics.

Tim Wehr
Posttranslationally modified sites in proteins are identified generally by digestion of the protein and identification of its constituent peptides. The low abundance of a particular posttranslationally modified species is a major impediment to its characterization in a complex mixture such as a body fluid or cell lysate. To obtain sufficient material for study and to eliminate the vast majority of interfering proteins, selective enrichment techniques are required. Enrichment can be performed at the protein level, or, more commonly, at the peptide level after enzymatic cleavage of the protein. Enrichment techniques for glycoproteins include lectin chromatography (1–3), size exclusion chromatography (4), hydrazide-based covalent coupling (5), and (in the case of sialic acid–containing glycopeptides) cation-exchange chromatography (6). Enrichment techniques for phosphoproteins and phosphopeptides were reviewed in the June issue of "Directions in Discovery" (7). These include immunoprecipitation (8,9), chemical modification by β-elimination (10) or using phosphoramidate chemistry (11), immobilized metal affinity chromatography (IMAC) (12), and enrichment on metal oxides such as titanium dioxide (13) or zirconium dioxide (14). Currently, IMAC or metal oxides are the most popular methods for enrichment of phosphopeptides.
The available enrichment techniques for phosphopeptides have two limitations. First, they tend to yield phosphopeptides as a class, with little or no discrimination among peptides differing in the level of phosphorylation. Second, no single enrichment technique delivers the entire phosphoproteome. A comparative study of phosphoramidate chemistry, IMAC, and TiO2 enrichment (15) demonstrated that overlap of the three techniques was poor, suggesting that each technique isolates a different subset of the phosphoproteome. The study of proteomics would benefit from a technique that would resolve phosphopeptides with different levels of phosphorylation and that would provide better (or complementary) representation of the phosphoproteome. Recently, electrostatic repulsion–hydrophilic interaction chromatography (ERLIC) has emerged as a candidate technique. ERLIC is a variation of hydrophilic interaction chromatography (HILIC) that shows promise for separation and enrichment of phosphopeptides (16) and glycopeptides (17).
Hydrophilic Interaction Chromatography
HILIC describes a type of normal-phase chromatography that employs a polar stationary phase with aqueous–organic mobile phases (18). In contrast to reversed-phase chromatography, the aqueous component of the mobile phase (for example, water or buffer) serves as the strong solvent, and the organic component (for example, acetonitrile) is the weak solvent. Analytes are eluted in order of increasing hydrophilicity. It has been proposed that retention occurs as the analyte partitions between the bulk mobile phase and a water-enriched layer which hydrates the hydrophilic stationary phase. This is in contrast to retention in conventional normal-phase chromatography, which occurs by adsorption at the polar stationary phase surface. To a first approximation, the retention order of a series of analytes in HILIC is the opposite of that in reversed-phase chromatography. HILIC has several advantages. First, it provides a means of achieving retention and separation of very hydrophilic water-soluble analytes, which are intractable to reversed-phase and ion-exchange chromatography. Second, the acetonitrile typically used as the organic component of the mobile phase has low UV transparency (for better detection sensitivity) and low viscosity (for high chromatographic efficiency). Third, a mobile phase composed of a low fraction of water (strong solvent) and a large fraction of acetonitrile (weak solvent) provides an ideal composition for efficient desolvation in electrospray ionization mass spectrometry (MS). HILIC has been used successfully for a wide variety of small molecule pharmaceuticals, carbohydrates, and peptides (19). It has been less successful for proteins because of their generally poor solubility in high concentrations of organic solvents. An exception is the application of HILIC to the separation of histones and membrane proteins.

Figure 1
Very basic and very hydrophilic species exhibit strong retention in HILIC, and, when present in a multicomponent mixture of less polar analytes, require gradient elution for separation within a reasonable time frame. The technique of ERLIC provides an alternative strategy for dealing with complex mixtures with components at the extreme of retention (poorly vs. highly retained).
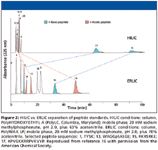
Figure 2
Electrostatic Repulsion–Hydrophilic Interaction Chromatography
Hydrophilic interaction can be superimposed as a mixed mode on an ion-exchange column in the presence of high concentrations of an organic solvent. The important feature of this combination is the independence of hydrophilic interaction and electrostatic effects, allowing the two to be manipulated independently. In this way, the behavior of charged solutes can be controlled to address extremes of retention. Three cases can be considered. The first case is the late elution of very acidic peptides containing aspartate or glutamate residues on an anion-exchange column. In this case, reduction of mobile phase pH below the pKa of the side chains reduces their retention. The second case is the strong retention of basic peptides on a HILIC column by hydrophilic interaction. In this case, use of an anion-exchange column in HILIC mode reduces retention by electrostatic repulsion of the basic groups from the stationary phase. The third case is the elution of basic peptides in the void volume of an anion-exchange column operated in anion-exchange mode. In this case, addition of sufficient organic solvent to the mobile phase introduces a HILIC contribution to the separation, and the retention of basic peptides is increased by hydrophilic interactions, which counteract the electrostatic repulsion of basic groups. In this case, the two superimposed modes antagonize each other's extremes of retention and can permit isocratic elution of complex peptide mixtures. This last case forms the basis for ERLIC of peptides (16).

Figure 3
The issue addressed in the third case is illustrated in Figure 1, in which a weak-anion-exchange column was operated in ion-exchange mode. Vasopressin has a net positive charge, is repelled electrostatically, and is eluted before the void volume since it is excluded from the pore system. The comparative performance of HILIC and ERLIC to resolve a mixture of peptide standards is shown in Figure 2. In HILIC mode, a concentration of acetonitrile that promotes retention of the basic peptides 15 and 17 affords inadequate retention of acidic and neutral peptides. In the ERLIC mode, electrostatic repulsion of peptides 15 and 17 allows the acetonitrile concentration to be increased to a level where all peptides can be retained and resolved.

Figure 4
ERLIC Isolation of Phosphopeptides
Most tryptic peptides carry at least two positive charges, one on the N-terminal amino group and the other on the C-terminal lysine or arginine residue. In anion-exchange chromatography, these positive charges dominate the chromatography, and nonphosphorylated peptides (represented as peptide A in Figure 3) are eluted within the void volume. The presence of a single phosphate residue (which reduces the net positive charge of the peptide by one unit) is inadequate to promote retention of the peptide in the anion-exchange mode, which still is eluted near the void volume (represented by peptide B in the lower trace of Figure 3). Addition of acetonitrile to the mobile phase drives the hydrophilic interaction of the phosphate with the stationary phase, promoting retention of peptide B (ERLIC mode, upper trace of Figure 3). Peptides with two or more phosphates are well-retained in either mode. Similar behavior can be seen with mono- and tetraphosphorylated peptides in a tryptic digest of β-casein (Figure 4).
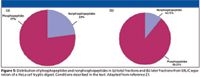
Figure 5
The selectivity of ERLIC for phosphopeptides under conditions where unphosphorylated peptides are unretained suggests that the technique could be an effective means of separating and enriching phosphopeptides from complex mixtures in proteomics applications. This was investigated using a tryptic digest of HeLa cell proteins (20,21). For such a complex sample, which could potentially contain thousands of phosphopeptides with variable levels of phosphorylation, gradient elution was required to elute mono- and multi-phosphorylated peptides within a reasonable time frame. In the study summarized in Figures 5–7, the digest was separated using a weak-anion-exchange column and the same solvents used in Figures 3 and 4 for standard peptides. The initial solvent was 20 mM sodium methylphosphonate (pH 2.0) containing 70% acetonitrile. An initial 10-min isocratic elution with this solvent was used to elute the poorly retained unphosphorylated peptides. Then a gradient to 200 mM triethylamine phosphate (TEAP, pH 2.0) containing 60% acetonitrile was performed. Note that this is a gradient in both the HILIC mode (decreasing acetonitrile) and the ion-exchange mode (TEAP is a stronger eluent than sodium methylphosphonate for phosphate groups). The low mobile phase pH served to uncharged aspartate and glutamate residues (suppressing their ion-exchange interactions), and to fully charge amine groups (promoting electrostatic repulsion). Fractions were collected across the elution time and each fraction was analyzed by reversed-phase liquid chromatography (LC)–MS-MS to identify phosphopeptides. As expected, unphosphorylated peptides eluted mainly in the early fractions, while phosphorylated peptides were retained and eluted with increasing concentration of TEAP (Figures 5 and 6). Moreover, separation based upon phosphorylation states was possible, with monophosphorylated peptides eluted first and multiply phosphorylated peptides eluted later in the gradient (Figures 7 and 8a). The number of phosphopeptides identified in the HeLa cell lysate compared favorably with the best results reported using titania or IMAC for enrichment.

Figure 6
A determination of the relative ratios of peptides phosphorylated on serine, threonine, or tyrosine residues (Figure 8b) revealed that 6% of the total was phosphorylated on tyrosine residues, which is significantly higher than previously reported values of ~2% (22). This might reflect the facts that ERLIC is more efficient in enriching multiphosphorylated peptides, and that 76% of the pY residues were found on such peptides.

Figure 7
ERLIC Enrichment and Separation of Glycopeptides
Based upon their observation that glycopeptides, like phosphopeptides, are retained poorly in strong cation chromatography using salt gradient elution, Lewandroski and colleagues (17) concluded that ERLIC conditions might be applied successfully to glycopeptide enrichment. They applied the technique to enrichment of tryptic glycopeptides derived from human plasma membrane proteins that had been extracted from platelets by PEG-dextran two-phase partitioning. The ERLIC separation conditions were identical to those described previously for phosphopeptide separations (PolyWAX weak-anion-exchange column using gradients from high to low acetonitrile concentration and from methylphosphonic acid to TEAP at low pH). Fractions collected during ERLIC elution were digested with PNGase, which removed N-linked oligosaccharides and introduced a +1 Da mass shift by deamidation. Subsequent LC–MS-MS was used to distinguish glycopeptides from nonglycopeptides based upon the deamidation mass shift and to identify the glycoproteins from which the glycopeptides were derived. As anticipated, nonglycopeptides were eluted in the early fractions, while the deamidated glycopeptides were eluted in later fractions (Figure 9). Most of the detected glycopeptides were eluted as broad peaks, with elution times of 5–6 min or greater. The authors interpreted this as a reflection of microheterogeneity at the glycosylation sites resulting in the separation of peptide isoforms. They conclude that ERLIC provides a promising means of enriching glycopeptides from complex peptide mixtures with the potential for resolving glycopeptide isoforms. The separation appears to be based upon hydrophilic interactions of carbohydrate groups, with a contribution from electrostatic interaction of sialic acid groups (pKa ~2.6) with the anion exchange phase. Possible interference by coeluted phosphopeptides could be eliminated by phosphatase treatment of the sample before ERLIC separation. Nearly half the deamidated peptides identified did not fit the consensus sequence for a glycation site. These might be peptides containing isoaspartic acid residues (pKa ~3.1) resulting from nonenzymatic deamidation.
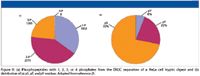
Figure 8
Conclusions
Comprehensive analysis of phosphopeptides or glycopeptides is hampered by the failure of any single enrichment technique to completely represent the total population of posttranslationallymodified species. Moreover, enrichment techniques tend to yield PTMs as a bulk mixture, with no resolution based upon PTM structure or site occupancy. ERLIC, a new technique in which the selectivities of HILIC and anion exchange are overlaid in the same separation, has been applied to the enrichment of both phosphopeptides and sialylated glycopeptides. In addition to serving as an enrichment tool, ERLIC can provide resolution based upon the number of modified sites (phosphopeptides) or the structure of the modified site (glycopeptides). These characteristic make ERLIC a promising alternative or complementary approach to phospho- and glycopeptides analysis.
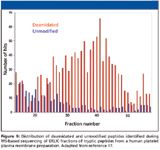
Figure 9
Acknowledgment
The author would like to thank Andy Alpert for providing figures for this column, and for discussion in its preparation.
Tim Wehr
"Directions in Discovery" editor Tim Wehr is staff scientist at Bio-Rad Laboratories, Hercules, California. Direct correspondence about this column to "Directions in Discovery," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgcmag.com.
References
(1) J. Ji, A. Chakraborty, M. Geng, X. Zhang, A. Amini, M. Bina, and F. Regnier, J. Chromatogr. 745, 197 (2000).
(2) M. Geng, J. Ji, and F.E. Regnier, J. Chromatogr., A 870, 295 (2000).
(3) M. Geng, X. Zhang, M. Bina, and F.E. Regnier, J. Chromatogr., B 752, 293 (2001).
(4) G. Alvarez-Manilla, J. Atwood 3rd, Y. Guo, N.L. Warren, R. Orlando, and M. Pierce, J. Proteome Res. 5, 701 (2006).
(5) H. Zhang, X.J. Li, D.B. Martin, and R. Aebersold, Nat. Biotechnol. 21, 660 (2003).
(6) U. Lewandrowski, R.P. Zahedi, J. Moebius, U. Walter, and A. Sickmann, Mol. Cell. Proteomics6, 1933 (2007).
(7) T. Wehr, LCGC 26(6), 550 (2008).
(8) S. Morandell, T. Stasyk, K Grosstessner-Hain, E. Roitinger, K. Mechtler, G.K. Bonn, and L.A. Huber, Proteomics6, 4047 (2006).
(9) F. Delom and E. Chevet, Proteome Sci. 4, 15 (2006).
(10) D.T. McLachlin and B.T. Chait, Anal. Chem. 75, 6826 (2005).
(11) H. Zhou, J.D. Watts, and R. Aebersold, Nat. Biotechnol. 19, 375 (2001).
(12) L. Andersson and J. Porath, Anal. Biochem. 154, 250 (1986).
(13) M.W. Pinkse, P.M. Uitto, M.J. Hilhorst, B. Ooms, and A.J. Heck, Anal. Chem. 76, 3935 (2004).
(14) H.K Kweon and K. Håkansson, Anal. Chem. 78, 1743 (2006).
(15) B. Bodenmiller, L.N. Mueller, M. Mueller, B. Domon, and R. Aebersold, Nature Methods 4, 231 (2007).
(16) A. Alpert, Anal. Chem. 80, 62 (2008).
(17) U. Lewandrowski, K. Lohrig, R. P. lZahedi, D. Wolters, and A. Sickmann, Clin. Proteom. DOI 10.1007/s12014-008-9007-z.
(18) A.J. Alpert, J. Chromatogr. 499, 177 (1990).
(19) P. Hemström and K. Irgum, J. Sep. Sci. 29, 1784 (2006).
(20) A. Alpert, G. Mitulovic, and K. Mechtler, poster P-2412-W, 32nd Ann. Symp. High Performance Liquid Phase Separations and Related Techniques, Baltimore (2008).
(21) G. Mitulovic, A. Alpert, and K. Mechtler, poster ThP 427, 56th ASMS Conference on Mass Spectrometry and Allied Topics, Denver, 2008.
(22) J.V. Olsen, B. Blagoev, F. Gnad, B. Macek, C. Kumar, P. Mortensen, and M. Mann, Cell 127, 635 (2006).


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









