Effects of Small Particle Size on SEC Characterization of Monoclonal Antibodies
A commercially available monoclonal antibody was analyzed via three different particle sizes (5 ?m, 3 ?m, and 2 ?m) to investigate the effects of particle size on resolution and the ability to increase sample throughput.
A commercially available monoclonal antibody was analyzed via three different particle sizes (5 µm, 3 µm, and 2 µm) to investigate the effects of particle size on resolution and the ability to increase sample throughput.
In the field of monoclonal antibodies (mAb), size exclusion chromatography (SEC) is the primary method used for analyzing high molecular weight aggregates. Carefully controlled, rigid, spherical silica particles with narrow particle distributions that are bonded with 1,2-dihydroxypropane are a mainstay of SEC analysis. These columns yield reliable and reproducible separation of mAbs and their aggregates. Recently, analytical scientists in search of greater sample throughput are seeking faster analysis times. Improvements in particle and column packing technology now allow companies to minimize analysis bottlenecks during product development and analysis of marketed products. This application note examines the impact of reducing particle size while increasing the speed of SEC separations and the resulting impact on resolution.
Experimental Conditions
Mobile Phase
100 mM sodium phosphate buffer at pH = 7.0 with 200 mM sodium chloride.
Instrument Parameters
HPLC System: Jasco X-LC
Flowrate: 1.0 mL/min
Column Temperature: 25 °C
Detection λ: 215 nm
Injection Volume: 5 µL for columns with 8.0 mm i.d.
1.5 µL for columns with 4.6 mm i.d.
Columns: YMC-Pack Diol, 5 µm, 200 Å, 8.0 × 300 mm
YMC-Pack Diol, 3 µm, 200 Å, 4.6 × 300 mm
YMC-Pack Diol, 3 µm, 200 Å, 4.6 × 150 mm
YMC-Pack Diol, 2 µm, 200 Å, 4.6 × 150 mm
Results
As seen in Figure 1, the flow rate was kept at 1.0 mL/min while column inside diameter was decreased (resulting in increasing linear velocity). As particle size was decreased, column length was shortened in order to negate increases in column back pressure. As linear velocity increased (due to the smaller i.d. columns) the analysis times were dramatically reduced while the smaller particle columns provided adequate resolution between monomer and aggregate(s). These results show that shorter columns can be used with smaller particles to increase throughput by greater than 6X while providing resolution of ≥1.2 between monomer and aggregates for mAbs.
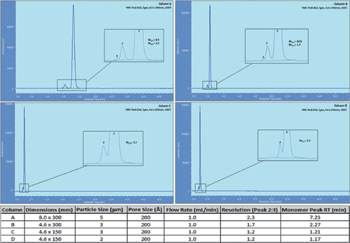
Figure 1: Separation of monomer and aggregate(s) on four different YMC-Pack Diol columns. Column dimensions and particle sizes can be seen in the table.
Conclusions
YMC-Pack Diol SEC columns in 3 µm particle size are an excellent choice for analyzing large-molecule protein samples under high-throughput conditions. The use of smaller particles for SEC analysis allows for column length to be shortened, increasing throughput, while simultaneously providing adequate resolution between monomer and aggregates in monoclonal antibody samples.
YMC America, Inc.
941 Marcon Blvd., Suite 201, Allentown, PA 18109
tel. (610) 266-8650, fax (610) 266-8652
Website: www.ymcamerica.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.














