Effects of Particle Size and Column Length on IEX Charge Variant Analysis of Monoclonal Antibodies
A commercially available monoclonal antibody was analyzed via two different particle sizes (5 ?m and 3 ?m) and column lengths (100 mm and 30 mm) to investigate their effects on resolution and the ability to increase sample throughput.
A commercially available monoclonal antibody was analyzed via two different particle sizes (5 µm and 3 µm) and column lengths (100 mm and 30 mm) to investigate their effects on resolution and the ability to increase sample throughput.
Ion exchange chromatography (IEX) is a powerful tool for evaluating charge variants in monoclonal antibody (mAb) drugs. The possibility of producing specific mAb isoforms can be seen as a way to improve the safety and efficacy profile of mAb therapeutics. The first step towards this goal is sufficient separation power of analytical methods using materials capable of separating as many of these charged isoforms as possible. YMC's new methacrylate-based strong cation exchange (SCX) stationary phase in 5 µm and 3 µm (non-porous) do not exhibit unwanted secondary interactions and allow for increased resolution in shorter columns providing faster run times and greater sample throughput.
Experimental Conditions
Mobile Phase
Analyses were run using 20 mM sodium phosphate buffer at pH = 6.5 with a 0–400 mM sodium chloride gradient over 55 min.
Instrument Parameters
HPLC System: Agilent 1100
Flow rate: 0.5 mL/min
Column Temperature: 25 °C
Detection λ: 215 nm
Injection Volume: 10 µL
Columns: YMC-BioProSP-F, 5 µm, 4.6 × 100 mm
YMC-BioPro SP-F, 3 µm, 4.6 × 30 mm Results
Columns packed with 5 µm SCX chemistry in a 100 × 4.6 mm format and 3 µm SCX chemistry in a 30 × 4.6 mm format were compared in order to demonstrate that a shift in particle size and a reduction in column length could serve to provide greater resolution in a shorter analysis time. As seen in Figure 1, a 30 × 4.6 mm 3 µm BioPro column shows an additional peak that was previously unresolved using the 100 × 4.6 mm 5 µm BioPro column, under the exact same method conditions (linear salt gradient of 0–400 mM over 55 min). Throughput was also increased by ~15%. The gradient was then shallowed (0–200 mM over 55 min) and resolution was further increased for all peaks.
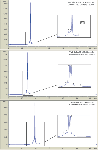
Figure 1: Charge variant separation of a monoclonal antibody on 5 µm and 3 µm YMC-BioPro SP-F columns. The shorter 3 µm column shows a previously unresolved component under the main peak. Resolution was further increased via a shallower 0â200 mM gradient.
Conclusions
YMC-BioPro non-porous SCX ion exchange columns in 5 µm and 3 µm particle size are an excellent choice for analyzing mAb charge variants. The use of smaller SCX particles in shorter columns for IEX analysis allows for greater resolution of charged isoforms and increased sample throughput. Milligram amounts of variants may be fractionated using 6 µm particles packed into semi-preparative sized columns.
YMC America, Inc.
941 Marcon Blvd., Suite 201, Allentown, PA 18109
tel. (610) 266-8650, fax (610) 266-8652
Website: www.ymcamerica.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.














