Developing High-Throughput Separations in Capillary GC
LCGC North America
Strategies for reducing analysis time in capillary gas chromatography
Moving to a faster analysis speed with capillary gas chromatography (GC) methods doesn't always require a new or upgraded instrument. A modest increase in analysis speed of two to four times shorter retention using conventional capillary columns with inner diameters of 150–200 μm can be achieved quite reasonably on a wide range of conventional laboratory instruments.
Typically, this involves reducing column length and internal diameter and optimizing the linear velocity for the carrier gas being used. A 30 m × 0.32 mm column will generate approximately the same number of theoretical plates as a 10 m × 0.1 mm column. The relationships between retention, resolution, and column geometry are well understood and software calculators are available to help scale column geometry and carrier velocity while retaining resolution (see the link to the full tutorial for details).
The fastest separations typically are obtained using hydrogen carrier gas because it produces highly efficient peaks at increased linear velocity (>100 cm/s is typical). Hydrogen generators with built-in safety features are very convenient and have largely mitigated the risks associated with using hydrogen in the laboratory. When implementing a separation using temperature-programmed mode, one should use "constant flow" mode to ensure that the carrier linear velocity does not decrease because of increased gas viscosity at higher temperatures.
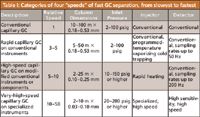
Table I: Categories of four "speeds" of fast GC separation, from slowest to fastest
There are several other important instrument considerations when translating to fast capillary GC methods, especially when using small internal diameter columns with hydrogen at high linear velocity. These include reducing system dead volume and sample diffusion; rapid introduction of sample from the inlet; rapidity of oven heating and cooling; and detector sampling rate.
The largest extracolumn volumes in a typical GC system will be the inlet liner and internal volume of the split–splitless inlet, and the void volume into which the column emerges in the detector.
Many operators choose to reduce the inlet dead volume by reducing the liner internal diameter and using a high split flow or use pulsed pressure injection to ensure the sample is transferred quickly and efficiently to the GC column — reducing the time available for diffusion of the sample plug within the inlet liner. Higher inlet pressures and split flows help to reduce the possibility for "backflash" of the sample vapors outside the inlet liner that gives rise to sample carryover.
In terms of the detector dead volume, one should ensure that the capillary column is installed according the manufacturer's guidelines because an incorrectly fitted column can cause catastrophic band broadening when using narrow internal diameter columns. Similarly, one needs to optimize the detector make-up gas flow rate (with flame-based detectors), as this gas assists with efficiently transferring the analyte into the flame region of the detector.
There are essentially two methods of obtaining very fast temperature programming rates in capillary GC: wrapping the capillary with electrically heated resistive tape, and using multicapillary bundles (~900 individual capillaries, ~40 μm i.d.) with heating wires inserted between the capillaries.
Although these systems are capable of very rapid heating (often >10 °C/s), the rate of heating such low-thermal-mass systems must be very well controlled to prevent retention time irreproducibility. High-boiling analytes may broaden as they are eluted isothermally at the upper temperature of the column.
Multicapillary bundle columns offer a higher total flow rate, higher sample capacity, and lower minimum detectable concentration of the solutes. Even though a speed increase of around 10-fold is possible with this approach, the technique is restricted to the analysis of fairly simple mixtures because of the short columns that typically are used.
Fast chromatography is characterized by narrow peaks with baseline elution profiles on the order of a few seconds to less than 1 s. To properly model the profile of the peak, ensure quantitative accuracy, and the optimum detector sensitivity, the detector sampling rate should be optimized.

Determining the Effects of ‘Quantitative Marinating’ on Crayfish Meat with HS-GC-IMS
April 30th 2025A novel method called quantitative marinating (QM) was developed to reduce industrial waste during the processing of crayfish meat, with the taste, flavor, and aroma of crayfish meat processed by various techniques investigated. Headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) was used to determine volatile compounds of meat examined.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










