Determination of Polycyclic Aromatic Hydrocarbons in Environmental Waters Using Heterogeneous Magnetic Materials Based on Metal–Organic Frameworks
LCGC North America
This study of PAHs makes it easy to compare this microextraction procedure to conventional approaches and to other procedures using magnetic composites.
Analytical chemistry efforts focus on the development of new extraction techniques that are faster and more sensitive than conventional approaches, normally incorporating novel materials in microextraction strategies with the ultimate purpose of following green analytical chemistry (GAC) requirements. Magnetic-facilitated microextraction methods using metal-organic frameworks as sorbents rise several GAC requirements.
In recent years, significant advances have been made in the development of more sensitive, simpler, and robust analytical methods for the monitoring of environmental contaminants (1,2). When dealing with highly complex samples, sample preparation approaches are sometimes avoided, despite the use of modern mass spectrometers (3). Therefore, efforts have been shifted to the implementation of simple, fast, and easily automated microextraction methods for proper sample preparation before the analytical determination (1,4). Among those methods is the miniaturized version of solid-phase extraction (µSPE), which requires low amounts of sorbents (<500 mg) (5). When performed under dispersive conditions (D-µSPE), the strength of the interaction between the target contaminant and the sorbent can be increased through vigorous stirring (for example, by vortexing or exposing the sample to ultrasound energy), thus increasing the overall extraction efficiency of the microextraction method (6). That efficiency increases a step further through the incorporation of magnetic materials to the sorbent, or by using a magnetic sorbent itself, to set up a magnetic-based microextraction (M-D-µSPE) (7–9). The magnetic character of the sorbent facilitates the overall methodology enormously, because centrifugation and filtration steps are avoided (the use of an external magnet permits simple decantation of the aqueous solution and the elution solvent), thus decreasing extraction times and minimizing possible sources of error.
The most common magnetic sorbents in these approaches are magnetic nanoparticles (MNPs) composed of iron nanoparticles in the magnetite phase (Fe3O4) (9). Bare MNPs are minimally used because of their high reactivity and instability and are usually protected with coating phases or are functionalized (7). The most common protecting shell is a silica coating, generating Fe3O4@SiO2, and with post-functionalization generating Fe3O4@SiO2-NH2 or Fe3O4@SiO2-NH2-COOH materials, among others. These magnetic materials can be combined with other coatings, thus originating a variety of hybrid magnetic materials that are currently facilitating the implementation of magnetic-facilitated microextraction strategies in laboratories worldwide (4,10). In fact, various materials have been described as promising sorbents in M-D-µSPE when combined with MNPs of magnetite: molecularly imprinted polymers (MIPs), carbonaceous material (carbon nanotubes and graphene mainly), and recently, metal–organic frameworks (MOFs) (11,12).
MOFs are porous hybrid materials composed of metal ions and organic linkers, presenting the highest surface areas known (13). Their crystal structure provides them with high thermal and chemical stability. Two routes can be followed to ensure proper combination of MOFs and MNPs. One is the simple mixing of MNPs and the MOF (8,14), although this approach does not succeed with all types of MOFs. The second route is the in situ growth of MOF crystals over MNPs. In this approach, MNPs are synthesized and then mixed with MOF precursors (organic linker and metal ion) in a proper MOF growth medium (15,16) to ensure the formation of the hybrid material. The final magnetic composite obtained by this method is more stable and presents more-reproducible analytical performance than the composite obtained by simple mixing. In addition, composites obtained by in situ growth require less equilibration time (a few seconds) under the action of an external magnetic field within the extraction procedure. After the MNPs are anchored onto the crystal MOF surface, the resulting heterogeneous magnetic material presents interesting applicability in magnetic-based microextraction procedures (6).
This study describes a magnetic-facilitated microextraction procedure for the determination of conventional contaminants such as polycyclic aromatic hydrocarbons (PAHs) in environmental waters. By choosing PAHs as target contaminants, the magnetic-based method can be compared easily with other conventional approaches (17–20) and with other similar procedures using magnetic composites based on MOFs (8,21,22). Four heterogeneous magnetic composites based on MOFs are selected as magnetic sorbents in the M-D-µSPE procedure, followed by ultrahigh-pressure liquid chromatography (UHPLC) and fluorescence detection (FD). The four magnetic composites based on MOFs were Fe3O4@SiO2-NH2-COOH/ZIF-8, Fe3O4@SiO2-NH2-COOH/HKUST-1, Fe3O4@SiO2-NH2-COOH/MOF-74(Mg,Co), and Fe3O4@SiO2-NH2-COOH/MOF-74(Mg).
Experimental
Chemicals, Reagents, Materials, and Samples
A mix of PAHs in acetonitrile was supplied by Dr. Ehrenstorfer GmbH (Ausburg, Germany), containing 10 mg/L in each of the following fluorescent PAHs: naphthalene (N), acenaphthene (Ace), phenanthrene (Phe), anthracene (A), fluoranthene (Ft), pyrene (Py), benz[a]anthracene (BaA), chrysene (Chy), fluorene (Fl), benzo[b]fluoranthene (BbFt), benzo[a]pyrene (BaPy), dibenzo[a,h]anthracene (Di(ah)A), indene(1,2,3-cd)pyrene (I(1,2,3-cd)Py), benzo[ghi]perylene (B(ghi)Per), and benzo[k]fluoranthene (BkFt).
In several studies, individual PAHs were tested. Thus, BaA, BbFt, BaPy, Di(ah)A and I(1,2,3-cd)Py were supplied by Dr. Ehrenstorfer GmbH as individual standard solutions in acetonitrile with a concentration of 10 mg/L.
BkFt (99%) and BbFt (98%) were supplied as solids by Fluka Chemika.
The rest of PAHs (98%) were supplied as solids by Aldrich.
Working standard solutions were prepared weekly in acetonitrile (HiPerSolv Chromanorm liquid chromatography [LC] grade, purchased from VWR), and stored at 4 °C.
Ultrapure water was obtained using an A10 MilliPore water purification system.
Ethanol (pro-analysis grade) and ammonia for analysis (25%) were obtained from Panreac.
UHPLC mobile phases were filtered with 0.45-µm Durapore membrane filters supplied by Sigma-Aldrich.
Whatman 0.2-µm polyvinylidene fluoride (PVDF) syringe filters were obtained from GE Healthcare and were used to filter all samples and standards before any UHPLC injection.
Pyrex centrifuge tubes with dimensions of 10 cm x 1.6 cm and a volume of 15 mL, were used in the microextraction procedure.
A prism (5 × 2 × 2 cm) NdFeB magnet was obtained from ENES Magnesy Pawel Zientek.
PTFE solvothermal reactors composed and stainless steel autoclaves were supplied by Parr Instrument Company.
Iron(III) chloride hexahydrate (FeCl3·6H2O) (≥99%), iron(II) chloride tetrahydrate (FeCl2·4H2O) (≥99%), sodium hydroxide (≥98%), and hydrochloric acid (≥37%), all from Sigma-Aldrich, were required in the synthesis of Fe3O4 MNPs.
To coat MNPs, tetraethyl orthosilicate (TEOS) (98%), 3-aminopropyltriethoxysilane (APTES) (<98%), and succinic acid (<99%), were used, all of them obtained from Sigma-Aldrich.
The following reagents were used to synthesize the MOFs:
Zn(NO3)2·6H2O (98%), 2-methylimidazolium acid (99.9%), Cu(NO3)2·6H2O (98%), benzene-1,3,5-tricarboxylic acid (95%), Mg(NO3)2·6H2O (99%), Co(SO2)2·7H2O (98%), 2,5 dioxoterephthalic acid, and 99.9% HPLC-grade dimethylformamide (DMF), all provided by Sigma-Aldrich.
Instruments and Equipment
The separation of PAHs was carried out using a 1260 Infinity UHPLC system from Agilent Technologies, with quaternary pumps capable of pressures as high as 600 bar, using a 50 mm × 4.6 mm, 1.8-µm Zorbax Eclipse PAH column, also from Agilent Technologies. The column thermostat was kept at 30 °C. The instrument was equipped with a Rheodyne 7725i valve with a 5-µL injection loop. A 1260 Infinity multichannel fluorescence detector from Agilent Technologies was used. This detector is able to operate simultaneously with one excitation wavelength (λex) and four emission wavelengths (λem), or with four excitation wavelengths and one emission wavelength. The UHPLC–FD system was controlled with the C.01.04 version of the OpenLab CDS ChemStation software (Agilent).
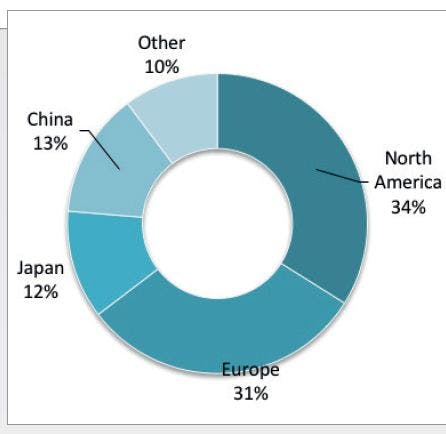
A linear gradient elution procedure was employed for the separation of the PAHs, using a mobile phase composed of acetonitrile–water at a constant flow rate of 1 mL/min and a gradient of 50–100% acetonitrile (v/v) in 11 min, with an isocratic hold at 100% acetonitrile for another 5 min. Table I includes the excitation and emission wavelengths, which were selected for the PAHs at each time interval (23).
A KM ultrasound bath (Shenzhen Codyson Electrical Co., Ltd.) was used in the microextraction procedure.
The microscopic morphology of the Fe3O4 and Fe3O4@SiO2-NH2-COOH/ZIF-8 materials was examined using a JSM6300 scanning electron microscope (JEOL), supporting the materials on a flat surface and using a cover with silver.
Synthesis of Magnetic Materials Based on MOFs
Selected MOFs were prepared according to previous reports: HKUST-1 (24), MOF-74(Mg) (25), MOF-74(Co,Mg) (25), and ZIF-8 (26). They were selected for their sorbent characteristics (high stability and adequate pore size) (27), and therefore they were used to prepare the magnetic composites. The preparation of the magnetic material follows three main stages: preparation of MNPs, applying the coating shell for MNPs, and in situ growth of the MOF onto the protected MNPs to obtain a heterogeneous magnetic material.
The first step is the synthesis of the magnetite nanoparticles. Fe3O4 MNPs were synthesized through an alkaline coprecipitation of Fe(II) and Fe(III), a well-known method developed by Kang and colleagues (28). The second step is the coating of the MNPs, according to previous reports of Kang and colleagues (29). Finally, the obtaining of Fe3O4@SiO2-NH2-COOH/MOF particles implies an in situ hydrothermal growth, including the MOFs precursors (metal and organic ligand, depending on each MOF), which are added in proper ratio over MNPs in DMF as proper solvent media. The final composite was characterized by scanning electron microscopy (SEM).
The preparation of Fe3O4@SiO2-NH2-COOH/ZIF-8 required Zn(NO3)2·6H2O (2 mmol) and 2-methylimidazolium acid (1 mmol); Fe3O4@SiO2-NH2-COOH/HKUST-1 required Cu(NO3)2·6H2O (2 mmol) and benzene-1,3,5-tricarboxylic acid (1 mmol); Fe3O4@SiO2-NH2-COOH/MOF-74(Mg) was synthesized with Mg(NO3)2·6H2O (3 mmol) and 2,5 dioxoterephthatic acid (1 mmol); and, finally, Fe3O4@SiO2-NH2-COOH/MOF-74(Mg,Co) required Mg(NO3)2·6H2O (1.5 mmol), Co(SO2)2·7H2O (1.5 mmol), and 2,5 dioxoterephthalatic acid (1 mmol).
Magnetic Dispersive Micro SPE
The optimum M-D-µSPE procedure used Fe3O4@SiO2-NH2-COOH/ZIF-8 as sorbent; 100 mg of this magnetic composite was added to 10 mL of aqueous standard or water sample containing PAHs. The mixture was subjected to 5 min of stirring using ultrasound. Afterwards, a magnet was placed outside the extraction tube for a few seconds, and the aqueous phase was simply removed. The magnetic composite containing the extracted PAHs was subjected to elution using 500 µL of acetonitrile and 5 min of ultrasound. The magnet was again placed outside the extraction tube, also for few seconds, and the eluate was easily collected with a syringe and injected in the UHPLC system.
Results and Discussion
Chromatographic Method
The separation of the 15 fluorescent PAHs was carried out by UHPLC–FD, using the wavelength program described in Table I. The successful separation of all 15 PAHs took less than 12 min.
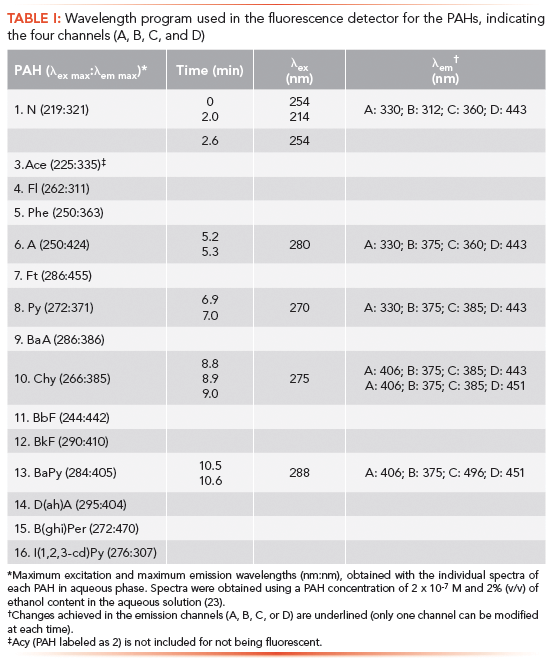
Table II shows several quality analytical parameters of the UHPLC–FD method. Calibration curves were linear, with correlation coefficient (R) values ranging from 0.994 to 0.999, for seven calibration levels. The limits of detection (LODs) and the limits of quantification (LOQs) were calculated at the signal-to-noise ratio (S/N) of 3 and 10, respectively, and were verified by preparation of standards at those levels. LODs ranged from 3 ng/L for Chy, BkFt, and BaPy, to 90 ng/L for I(1,2,3-cd)Py.
Two different standards were used to evaluate the precision of the chromatographic method. The concentration levels were within the calibration range for each PAH, but were different from those tested as the calibration levels (see Table II). In all cases, RSD values (interday precision in %) for the concentration levels indicated in Table II were lower than 10.9% for the higher concentration level tested. Regarding the reproducibility of the retention times, RSD values were always lower than 0.9% (n = 30, interday evaluation).
Optimization of the M-D-µSPE-UHPLC–FD Method
Most relevant variables exerting an influence in the M-D-µSPE method for PAH extraction were evaluated using "one factor at a time" optimization. The studied variables were the magnetic sorbent nature and amount, and the number of elution steps. Several other variables were fixed in the entire optimization to follow common microextraction strategies or to ensure adequate sensitivity: 10 mL for the water sample volume, 5 µg/L for the spiked PAHs concentration (a high level to ensure their detection despite nonoptimum conditions), 5 min of ultrasound exposure to ensure proper dispersion of the magnetic composite in water, 5 min of ultrasound during elution, acetonitrile as the elution solvent (8), and 500 µL of acetonitrile as the elution volume.
Screening of Fe3O4@SiO2-NH2-COOH/MOF Composites as Sorbents for PAHs in M-D-µSPE
Magnetic composites based on MOFs, described above, were tested in M-D-µSPE to screen their extraction efficiency for the PAHs. In these studies, an aqueous standard of PAHs (10 mL) was mixed with 20 mg of four different Fe3O4@SiO2-NH2-COOH/MOF materials, using the remaining fixed parameters mentioned above. The use of these types of magnetic composites yielded separation times of only a few seconds with the external magnet, as opposed to those approaches in which the MOF is not chemically linked to the magnetic nanoparticles (which required a few minutes of separation time) (8). The results are shown in Figure 1. The magnetic composite based on ZIF-8 exhibited better results, and thus it was selected to set up the magnetic-based method.
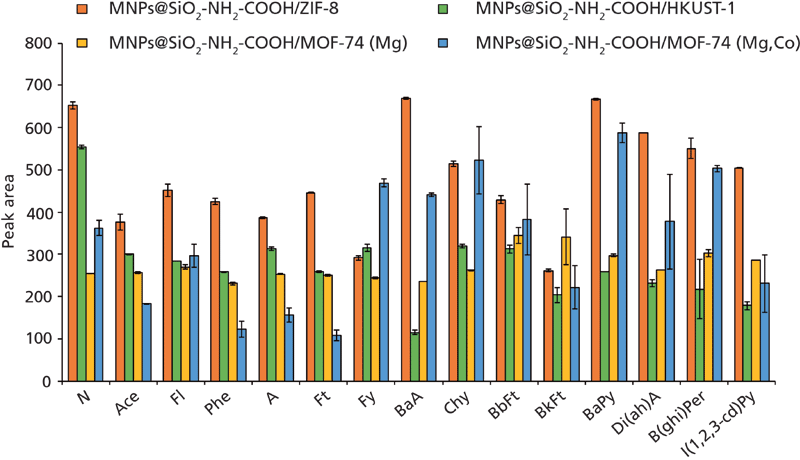
Figure 1: Screening study to evaluate the performance of different heterogeneous magnetic composites based on MOFs in a M-D-µSPE-HPLC-FD approach for determining PAHs. Specific experimental conditions are described in the text. Screening study to evaluate the performance of different heterogeneous magnetic composites based on MOFs in a M-D-µSPE-HPLC-FD approach for determining PAHs. Specific experimental conditions are described in the text.
Fe3O4@SiO2-NH2-COOH/ZIF-8 Amount Evaluation
The amount of Fe3O4@SiO2-NH2-COOH/ZIF-8 was optimized to obtain the highest extraction efficiencies with the lowest amount of sorbent. Figure 2 shows the relative amounts of PAHs extracted (monitored as peak area values), which were obtained using varying amounts of magnetic composite (from 5 to 100 mg), with the same group of fixed conditions. Clearly, the best results were obtained using 100 mg of Fe3O4@SiO2-NH2-COOH/ZIF-8. Judging from the results, higher amounts of material would show a higher extractive efficiency for PAHs. Nevertheless, higher amounts were neither tested nor selected, to ensure that a microextraction approach could be used in the environmental monitoring method pursued and to ensure proper dispersion of the sorbent in the sample.
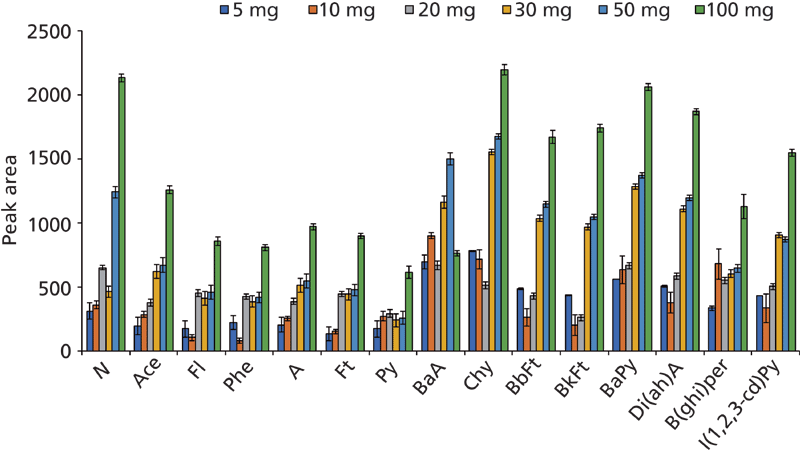
Figure 2: Study of the influence of MµPs@SiO2-NH2-COOH@ZIF-8 amount. The experimental conditions are described in the text.
Optimization of the Elution Step
Acetonitrile was selected as elution solvent because it provided adequate results for the elution of PAHs (8), and also given its compatibility with LC mobile phases. LC-incompatible solvents were not tested, thus avoiding the inclusion of an additional of step of solvent exchange, as well as possible losses of lighter PAHs. The number of elution steps was also evaluated (from one to four), and it was determined that 500 µL of acetonitrile was adequate to elute PAHs in the composites tested.
The optimum procedure of the M-D-µSPE-UHPLC-FD method for the determination of PAHs in water is shown in Figure 3.
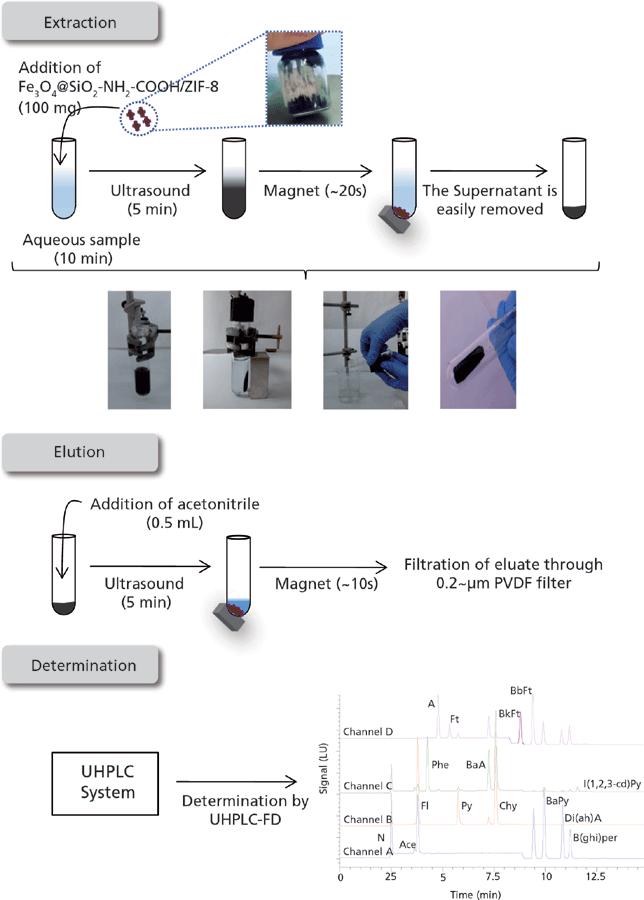
Figure 3: Optimum M-D-µSPE-UHPLC-FD method for determining PAHs in waters. Representative chromatogram using an aqueous standard (20 µg/L in PAHs). The multichannel program used for FD is described in the text.
Validation of the M-D-µSPE-UHPLC-FD Method
Table III includes several quality analytical parameters of the optimum M-D-µSPE-UHPLC-FD method, using Fe3O4@SiO2-NH2-COOH/ZIF-8 as sorbent. R values higher than 0.994 were obtained in all cases. LODs and LOQs were calculated as a signal-to-noise ratios of 3 and 10, respectively, and they were verified by preparation of aqueous standards at those levels. LODs ranged from 0.3 ng/L for Chy and BkFt to 13 ng/L for I(1,2,3-cd)Py. On average, the sensitivity achieved with the preconcentration obtained using the magnetic composite was 7.7 times higher than that obtained in absence of any enrichment procedure, and it was achieved in only ~10 min. The obtained LODs are comparable to those obtained in our previous study using a magnetic composite based on MOFs in which there was not any chemical linkage between the MOF (specifically HKUST-1) and the MNPs (8). In that study, only heavy PAHs (eight hydrocarbons) were tested, and LODs ranged from 0.8 to 4.6 ng/L also using M-D-µSPE-UHPLC–FD. However, the current method results much faster: ~10 min for the entire sample preparation time versus ~45 min in the previous study (8), and a higher number of PAHs can be determined in the current approach. Du and colleagues used magnetic core–shell particles based on MOFs (and thus with chemically attached MOF-MNPs), specifically Fe3O4@MAA@MIL-100 (where MAA is mercaptoacetic acid and MIL-100 is the MOF used), to determine 13 PAHs in water samples. The obtained LODs varied from 32 to 2110 ng/L (higher than the ones reported in the current study) using M-D-µSPE-HPLC-FD and relatively higher sample preparation times (~18 min) (21). Hu and colleagues used a heterogeneous magnetic material based on MOF ZIF-11 (a zeolite with a similar structure to that of ZIF-8), also in a M-D-µSPE-HPLC-FD approach, obtaining LODs ranging from 0.08 to 1.6 ng/L for heavy PAHs (seven hydrocarbons), while employing ~3 min for extraction and ~12 min for elution and solvent exchange (overall ~15 min) (22). Regarding conventional extraction, such as liquid–liquid extraction (LLE), Payanan and colleagues used acetonitrile–acetone as the extractant solvent, followed by an SPE clean-up step in combination with HPLC–FD, to determine 16 PAHs in edible oil samples. The LOD values varied from 0.25 to 6.25 µg/kg (20). Other researchers have employed dispersive liquid–liquid microextraction using 2-ethyl-1-hexanol as the extraction solvent and methanol as the dispersive solvent for the determination of five PAHs in various environmental waters. They have obtained adequate results, with LODs of 0.002–0.8 µg/L (comparable to those obtained in this study) (18).
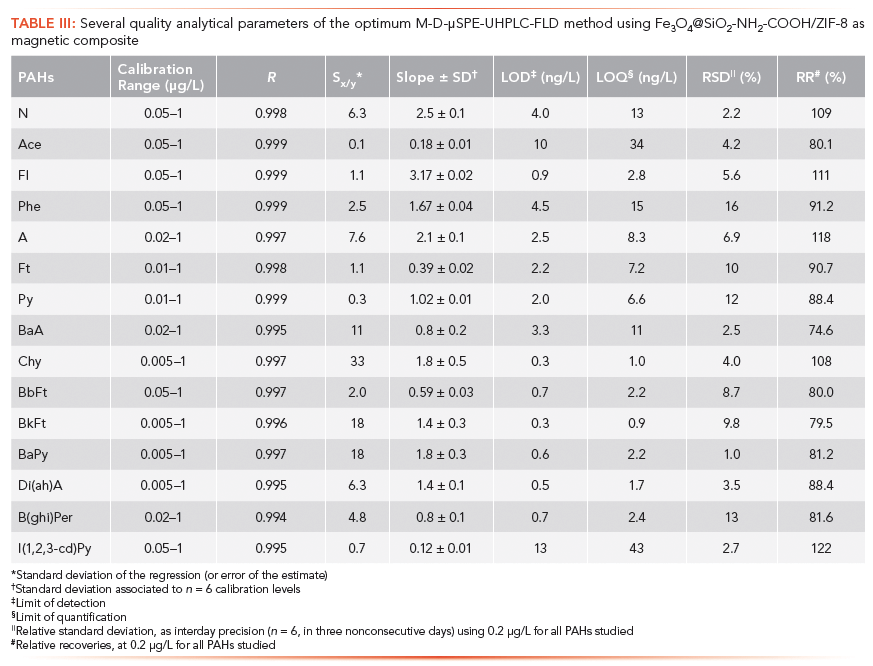
The performance of the M-D-µSPE-UHPLC-FD method has also been evaluated by assessing the precision as RSD values (RSD in %), and the relative recovery (RR, in %), with interday studies (n = 6, three nonconsecutive days), and using a spiked level of 0.2 µg/L (intermediate concentration value of the calibration curve). The results are shown in Table III. RR values varied from 74.6% to 122.4%, which is considered as adequate. RSD values were 1.0% for BaPy and 16% for Phe.
Considering their low water solubility and high affinity for particulate matter, PAHs are usually present in environmental waters at low levels. However, they constitute a potential carcinogenic and mutagenic risk for human health even at those levels. Therefore, several organizations have set regulation levels for these contaminants. The United States Environmental Protection Agency (EPA) has determined that the levels for BaPy in drinking water should not be higher than 0.2 µg/L (30). The European Union (EU) legislation (31) has set up regulation levels for seven PAHs, five of them termed as priority PAHs: BaPy, BbFt, BkFt, B(ghi)Per, and I(1,2,3-cd)Py. The maximum value set for BaPy is 0.1 µg/L in superficial and drinking waters. The sum of the contents of the remaining priority PAHs is compared to that of BaPy as reference value, and thus the total concentration in superficial waters cannot exceed the limit of 0.1 µg/L. The EU has also set maximum concentration values for A and Fl of 0.4 µg/L and 1 µg/L, respectively.
Considering the values imposed by current legislation, it is clear that the proposed magnetic-based method is completely valid for the determination of PAHs (LOD of 2.5 ng/L for A, 0.9 ng/L for Fl, and 0.6 ng/L for BaPy, being the sum of LODs for BbFt, BkFt, B(ghi)Per, and I(1,2,3-cd)Py equal to 14.7 ng/L).
Conclusions
Magnetic composites based on MOFs have been used adequately in a magnetic-based D-µSPE method for determining PAHs in water. The magnetic composites can be separated easily from water by using an external magnet for few seconds. The magnetic composite based on the MOF ZIF-8, Fe3O4@SiO2-NH2-COOH/ZIF-8, demonstrated analytical extraction performance for PAHs superior to that of the rest of composites tested.
The optimum magnetic-based method provides the following benefits: short sample preparation times (10 min), simplicity (mixing and ultrasound as sample preparation steps), and adequate sensitivity when combining the M-d-µSPE method with UHPLC–FD, which allows it to reach limits of detection as low as 0.3 ng/L (thus fulfilling environmental monitoring requirements). The entire method also presents quite adequate interday reproducibility (<16%) and average efficiencies (93.6%), while requiring low amounts of sorbents (100 mg) and low organic solvent consumption (500 µL of acetonitrile in the extraction step).
Acknowledgments
V.P. and C.R.-P. thank the Spanish Ministry of Economy and Competitiveness (MINECO) for the Project Ref. MAT2014-57465-R. P.R.-B. thanks her FPI PhD research contract associated to the Project Ref. MAT2014-57465-R. J.P. thanks the funds from the Cabildo de Tenerife under the 'Agustín de Betancourt' Program.
References
(1) A. Spietelun, Å. Marcinkowski, M. Guardia, and J. Namiesnik, J. Chromatogr. A 1321, 1–13 (2013).
(2) H. Yan and H. Wang, J. Chromatogr. A 1295, 1–15 (2013).
(3) K.M. Dimpe and P.N. Nomngongo, Trends Anal. Chem. 82, 199–207 (2016).
(4) B. Henrique-Fumes, M. Ribeiro-Silva, F. Nascimento-Andrade, C.E. Domingues-Nazario, and F. Mauro-Lanças, Trends Anal. Chem. 71, 9–25 (2015).
(5) J. PÅotka-Wasylka, N. Szczepasnka, M. Guardia, and J. Namiesnik, Trends Anal. Chem. 73, 19–38 (2015).
(6) P. Rocío-Bautista, P. González-Hernández, V. Pino, J. Pasán, and A.M. Afonso, Trends Anal. Chem. 90, 114–134 (2017).
(7) P. Rocío-Bautista and V. Pino, Analytical Separation Science; Sample Preparation, Method Validation, and Analytical Applications Vol. 5 (Wiley VCH, Weinheim, Germany, 2015), Chapter 11, pp. 1681–1724. .
(8) P. Rocío-Bautista, V. Pino, J.H. Ayala, J. Pasán, C. Ruiz-Pérez, and A.M. Afonso, J. Chromatogr. A 1436, 42–50 (2016).
(9) Á. Ríos and M. Zougagh, Trends Anal. Chem. 84, 72–83 (2016).
(10) J. Tian, J. Xu, F. Zhu, T. Lu, C. Su, and G. Ouyang, J. Chromatogr. A 1300, 2–16 (2013).
(11) J. PÅotka-Wasylka, N. Szczepasnka, M. Guardia, and J. Namiesnik, Trends Anal. Chem. 77, 23–43 (2016).
(12) Y. Wen, L. Chen, J Li, D. Liu, and L. Chen, Trends Anal. Chem. 59, 26–41 (2014).
(13) O.M. Yaghi, M. O'Keeffe, N.W. Ockwig, H.K. Chae, M. Eddaoudi, and J. Kim, Nature 423, 703–714 (2003).
(14) J. Ma, Z. Yao, L. Hou, W. Lu, Q. Yang, J. Li, and L. Chen, Talanta 161, 686–692 (2016).
(15) Y. Wang, J. Xie, Y. Wu, H. Ge, and X. Hu, J. Mater. Chem. A 1, 8782–8789 (2013).
(16) Y. Hu, Z. Huang, J. Liao, and G. Li, Anal. Chem. 85, 6885-6893 (2013).
(17) L.L.A. Veiga, H. Amorim, J. Moraes, M.C. Silva, R.S.L. Raices, and S.L. Quiterio, Food Chem. 152, 612–618 (2014).
(18) M.H. Fatemi, M.R. Hadjmohammadi, P. Shakeri, and P. Biparva, J. Sep. Sci. 35, 86–92 (2015).
(19) L. Foan and V. Simon, J. Chromatogr. A 1256, 22–31 (2012).
(20) T. Payanan, N. Leepipatpiboon, and P. Varanusupakul, Food Chem. 141, 2720–2726 (2013).
(21) F. Du, Q. Qin, J. Deng, G. Ruan, X. Yang, L. Li, and J. Li, J. Sep. Sci. 39, 2356–2364 (2016).
(22) H. Hu, S. Liu, C. Chen, J. Wang, Y. Zou, L. Lin, and S. Yao, Analyst 139, 5818–5826 (2014).
(23) J.H Ayala, A.M Afonso, and V. González, Appl. Spectrosc. 51, 380–386 (1998).
(24) Q.M. Wang, D. Shen, M.L. Lau, S. Deng, F.R. Fitch, N.O. Lemcoff, and J. Semanscin, Micropor. Mesopor. Mat.55, 217–230 (2002).
(25) L.J. Wang, H. Deng, H. Furukawa, F. Gándara, K.E. Cordova, D. Peri, and O.M. Yaghi, Inorg. Chem. 53, 5881-5883 (2014).
(26) D. Ge and H.J. Lee, J. Chromatogr. A 1263, 1–6 (2012).
(27) P. Rocío-Bautista, C. Martínez-Benito, V. Pino, J.H. Ayala, J. Pasán, C. Ruiz-Pérez, and A.M. Afonso, Talanta, 139 13–20 (2015).
(28) Z. Wang, H. Guo, Y. Yu, and N. He, J. Magn. Magn. Mater. 302, 397–404 (2006).
(29) Y.S. Kang, S. Risbud, J.F. Rabolt, and P. Stroeve, Chem. Mater. 8, 2209–2211 (1996).
(30) D. Lerda, European Commission, Institute for Reference Materials and Measurements (2011).
(31) Decision No 2455/2001/EC of the European parliament and of the council of 20 November 2001, establishing the list of priority substances in the field of water policy and amending Directive 2000/60/EC Official Journal of the European Union, L331/1.
Priscilla Rocío-Bautista, Verónica Pino, Juan H. Ayala and Ana M. Afonso are with the Department of Chemistry (Analytical Division), University of La Laguna, Tenerife, Spain.
Jorge Pasán and Catalina Ruiz-Pérez are with the X Ray and Molecular Materials Lab (MATMOL), Physics Department, University of La Laguna, Spain.
Direct correspondence to: veropino@ull.edu.es

A Novel LC–QTOF-MS DIA Method for Pesticide Quantification and Screening in Agricultural Waters
May 8th 2025Scientists from the University of Santiago de Compostela developed a liquid chromatography quadrupole time-of-flight mass spectrometry (LC–QTOF-MS) operated in data-independent acquisition (DIA) mode for pesticide quantification in agriculturally impacted waters.
Distinguishing Alcohol- from Non-Alcohol-Associated Liver Cirrhosis with LC-MS
May 7th 2025A pilot study investigating whether nicotinamide adenine dinucleotide kinase (NADK) expression is selectively diminished in alcohol-associated liver cirrhosis (AC), as well as evaluating its potential as a biomarker for this condition, measured AC and non-AC (NAC). Nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADP+) levels in human liver samples were measured using liquid chromatography-mass spectrometry (LC-MS).
University of Oklahoma and UC Davis Researchers Probe Lipidomic Profiles with RP-LC–HRMS/MS
May 6th 2025A joint study between the University of Oklahoma Health Sciences Center (Oklahoma City, Oklahoma) and the UC Davis West Coast Metabolomics Center (Davis, California) identified differentially regulated lipids in type 2 diabetes (T2D) and obesity through the application of reversed-phase liquid chromatography-accurate mass tandem mass spectrometry (RP-LC-accurate MS/MS).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)