Determination of Methacholine Chloride and Potential Impurities Using a Reagent-Free Ion Chromatography System
Methacholine chloride is a synthetic analogue of the neurotransmitter acetylcholine used to assess bronchial asthma of subjects in respiratory function labs and epidemiological field studies (1).
Richard Kornfeld and Jeffrey Rohrer, Dionex Corporation
Methacholine chloride is a synthetic analogue of the neurotransmitter acetylcholine used to assess bronchial asthma of subjects in respiratory function labs and epidemiological field studies (1). Methacholine has been shown to decompose over time (2). Hydrolysis will yield β-methylcholine and acetic acid. Acetylcholine, a potential synthetic impurity, may also be present in the powder.
Choline and its analogs have been determined using ion chromatography (IC) in various matrices such as prescription and nonprescription medications, milk and infant formula, and vitamin and mineral formulations (3 and references cited therein). This contribution demonstrates that a cation RFIC system can determine methacholine, acetylcholine, and β-methylcholine with high precision, high recovery, and excellent day-to-day reproducibility. We use anion IC to determine acetate. The first method can assay methacholine, and the combination of both methods can determine methacholine's related substances.
Experimental
Analyses are performed using a Dionex ICS-2100, AS Autosampler, and Chromeleon® Chromatography Data System software. Cation separations use an IonPac® CG/CS17 column set with electrolytically generated MSA eluent, while anion separations use an IonPac AG/AS22 column set with manually prepared carbonate/bicarbonate eluent. For detailed experimental conditions, see Dionex Application Note 249.
Results and Discussion
The method for methacholine assay includes diluting the initial solution (25 g/L methacholine in 0.9% sodium chloride with or without 0.4 % phenol) 1000-fold with water. Excellent linearity (R2 > 0.9999), spike recovery (97–100%), 5-day retention time (<0.05 %), and peak area (<0.4 %) RSDs were observed for methacholine.
The method for potential methacholine impurities includes diluting the initial methacholine solution 100-fold with water. Figure 1 shows the cation IC of 2.5 mg/L acetylcholine in diluted methacholine-containing matrix. A peak for β-methylcholine is observed in both the matrix blank and acetylcholine-spiked matrix at approximately 0.1% of the methacholine concentration. MDL estimates for β-methylcholine and acetylcholine are at single-digit μg/L levels while the estimate for acetate MDL is 75 μg/L. Excellent linearity (R2 > 0.9998) for target impurities are observed. Recoveries of target impurities spiked at 2.5 mg/L range from 85–95%. Highly reproducible retention times (0.1 % or less) and peak areas (< 0.8%) are observed for the choline-based analytes over a 5-day study.
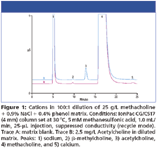
Figure 1
Conclusions
This note presents IC-based methods for the detection and quantification of methacholine and potential impurities in solutions used to assess bronchial asthma. These assays only require simple dilutions with deionized water and demonstrate excellent precision and accuracy for both methacholine and for potential impurities present in amounts approaching 1%.
References
(1) Guidelines for Methacholine and Exercise Challenge Testing – 1999. Am. J. Respir. Crit. Care Med., 161, 309–329 (2000).
(2) R. D. Hayes, et. al., Eur. Respir. J., 11, 946–948 (1998).
(3) Dionex Corporation. Application Note 194 (LPN 1967, April, 2008).
Chromeleon and IonPac are registered trademarks of Dionex Corporation.

Dionex Corporation
1228 Titan Way, P.O. Box 3603, Sunnyvale, CA 94088
tel. (408)737-0700; fax (408)730-9403
Website: www.dionex.com

Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.



















