Determination of Fatty Acid Esters of 2- and 3-Monochloro-1,2-propanediol (MCPD) and Glycidol in Edible Oil Using GC–MS TQ
The Application Notebook
In the present study, a novel GC–MS TQ method was developed and validated for the simultaneous analysis of 2-MCPD, 3-MCPD, and glycidyl fatty acid esters in edible oil. This method was subsequently applied to quantitation of these contaminants in commercial edible oil samples.
Esterified and free forms of 2-MCPD, 3-MCPD, and glycidol are heat-induced contaminants found in various types of processed food (1). Numerous studies have investigated the levels of these contaminants in oils and fats from different sources, and mitigation strategies are currently under development. However, to support future research where higher sensitivity and selectivity are necessary, analytical solutions based on triple-quadrupole mass spectrometers will be required. In the present study, a novel GC–MS TQ method was developed and validated for the simultaneous analysis of 2-MCPD, 3-MCPD, and glycidyl fatty acid esters in edible oil. This method was subsequently applied to quantitation of these contaminants in commercial edible oil samples.
Methods and Materials
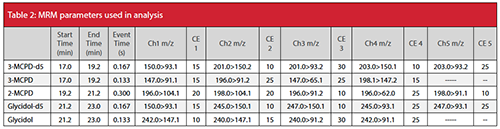
Fatty acid esters of 2-MCPD, 3-MCPD, and glycidol in oil were extracted and derivatized using phenylboronic acid according to the AOCS Official Method Cd 29a-13 (2). The analyte standards used were 1,3-dipalmitoyl-2-chloropropanediol (2-MCPD), 1,2-dipalmitoyl-3-chloropropanediol (3-MCPD), and glycidyl palmitate (GlyP). The internal standards were 1,2-dipalmitoyl-3-chloropropanediol-d5 (3-MCPD-d5) and glycidyl palmitate-d5 (GlyP-d5). The extracts were analyzed on a GCMS-TQ8040 NX system (Shimadzu Corporation). Separation was performed using a 30 m × 0.25 mm, 1.0-μm SH-Rxiâ1MS capillary column (Shimadzu Corporation). Detailed instrumental conditions are presented in Table 1, and MRM parameters for the different analytes are shown in Table 2.
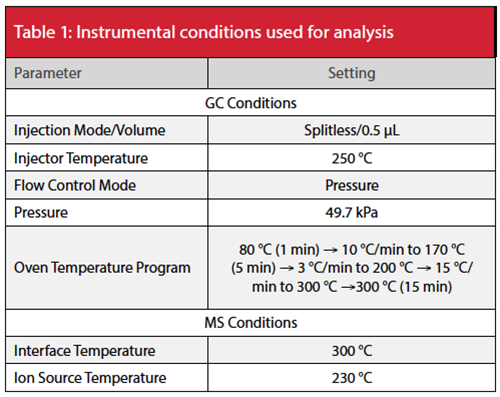
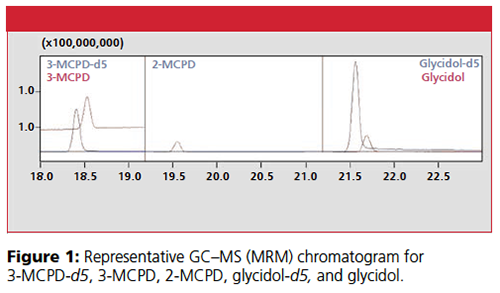
Results
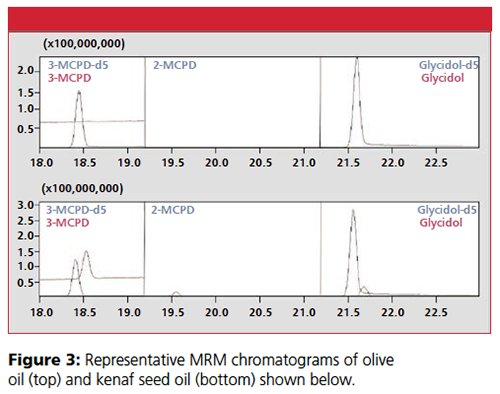
MRM chromatograms of the analytes (using target MRM transitions) showed good peak shapes (Figure 1). The retention times of 3-MCPD-d5, 3-MCPD, 2-MCPD, glycidol-d5, and glycidol were 18.4 min, 18.6 min, 19.6 min, 21.6 min, and 21.7 min, respectively.
Method validation was performed to assess parameters such as sensitivity, accuracy, precision, linearity, and repeatability using calibration standards or quality control (QC) samples. Subsequently, this method was applied to quantitation of the different analytes in commercially available edible oil samples.
Sensitivity
The sensitivity of the assay was examined by measuring the signal to noise (S/N) ratio of the analyte peaks. The LOD (limit of detection) was defined as S/N ratio of at least 5. The LOD for this method was 0.003 μg of 2-MCPD and 3-MCPD and 0.006 μg of glycidol. The limit of quantitation (LOQ) was defined as S/N ratio of at least 10. The LOQ of this method was 0.01 μg of 2-MCPD and 3-MCPD and 0.024 μg of glycidol.
Accuracy and Precision
QC samples at three concentrations (QC1, QC2, and QC3) were used to investigate the accuracy and precision of the method. High accuracy and precision were demonstrated for this method as the accuracies of the QC samples were all within 100 ± 7% and the %RSD were all < 10% (n = 6).
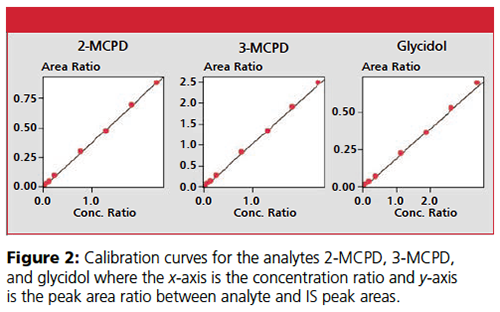
Linearity and Repeatability
Excellent repeatability of the peak areas of consecutive injections were achieved for all analytes (< 3%) (n = 6). Eight calibration standards for 2-MCPD and 3-MCPD ranging 0.010–0.930 μg and glycidol 0.024–2.130 μg were used to construct the calibration curves. The calibration curves (Figure 2) for all analytes showed excellent linearity (R2 > 0.998) (n = 6).
Application of Method for Quantitation of Analytes in Commercially Available Oil Samples
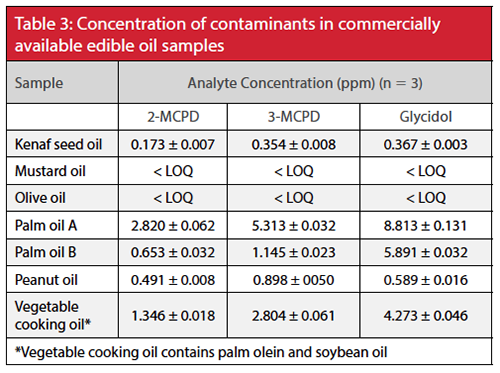
The validated method was applied to quantitation of esters of 2-MCPD, 3-MCPD, and glycidol in edible oil samples from different sources (Table 3, Figure 3). Generally, samples containing palm oil were found to contain the highest levels of the contaminants.
Conclusions
In summary, a novel GC–MS method was developed and validated for the simultaneous analysis of 2- and 3-MCPD and glycidol fatty acid esters in edible oil. This method showed more than threefold improvement in sensitivity compared to the official method currently available and demonstrated excellent linearity, repeatability, accuracy, and precision. Application of this method to the analyses of commercially available edible oil samples confirmed that samples containing palm oil show higher levels of contaminants.
References
- Federation for European Oil and Proteinmeal Industry, “FEDIOL Q&A on 2- and 3-MCPD and Their Esters and Glycidyl Esters”, (2016).
- The American Oil Chemists’ Society, “2- and 3-MCPD Fatty Acid Esters and Glycidol Fatty Acid Esters in Edi-ble Oils and Fats by Acid Transesterification. AOCS Official Method Cd29a-13, Cd29b-13 and Cd29c-13”, (2013).

Shimadzu Europa GmbH
Albert-Hahn-Str. 6–10,
D-47269 Duisburg, Germany
Tel.: +49 203 76 87 0
Fax: +49 203 76 66 25
E-mail: shimadzu@shimadzu.eu
Website: www.shimadzu.eu

Determining Enhanced Sensitivity to Odors due to Anxiety-Associated Chemosignals with GC
May 8th 2025Based on their hypothesis that smelling anxiety chemosignals can, like visual anxiety induction, lead to an increase in odor sensitivity, a joint study between the University of Erlangen-Nuremberg (Erlangen, Germany) and the Fraunhofer Institute for Process Engineering and Packaging (Freising, Germany) combined behavioral experiments, odor profile analysis by a trained panel, and instrumental analysis of odorants (gas chromatography-olfactometry) and volatiles (gas chromatography-mass spectrometry).
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










