Add More Confidence to Your UHPLC–MS Analysis
The Application Notebook
For your highly sensitive UHPLC–MS analyses, how can you reduce noise and additional signals to a minimum?
For your highly sensitive UHPLC–MS analyses, how can you reduce noise and additional signals to a minimum? Our new high-end UHPLC–MS solvents raise the standard for low baseline noise and clean mass spectra.
Our new range of advanced UHPLC–MS LiChrosolv® solvents have been developed to exceed all expectations, providing rapid and reliable results in both ESI/APCI positive and negative ionization modes.
Thanks to their lowest level of background noise and ion suppression, this quality ensures the optimum ionization efficiency to enable the highest sensitivity. With these features, use of these solvents can also help to extend column lifetime.
To ensure that you have confidence in your results, we specify the lowest possible limit of polyethylene glycol (PEG) impurities in all our UHPLC–MS solvents.
Our advanced UHPLC–MS LiChrosolv solvents have been designed to meet the highest requirements of UHPLC–MS in research and quality control, including proteomics and metabolomics, as well as environmental, clinical, food, or industrial testing applications.
A new standard for the unlimited application of ultrahigh-pressure chromatography has been set.
Features and Benefits
- Suitability tested and specified for UHPLC–MS and UHPLC–UV: for analytical flexibility
- Specified quality in positive and negative ESI and APCI MS for lowest detection limits and confidence in analyses in all important MS modes (Test 1)
- ESI or APCI ({+) < 2 ppb
- ESI or APCI (-) < 10 ppb
- Lowest impurity profile: for interference-free baselines (Test 2)
- Microfiltration through 0.2 µm filter (Test 3)
- Prolonged lifetime of filters and mechanical parts in HPLC systems
- Reduced risk of column clogging
- Packaged in borosilicate glass bottles: minimized contamination with metal ions
- Lowest levels of trace metal impurities: for minimized metal ion adduct formation
- <5 ppb
- Lowest level of polyethylene glycol (PEG) impurities in our entire UHPLC–MS solvent lineup to give you confidence in your results (PEG S/N signal-to-noise-ratio < 50)
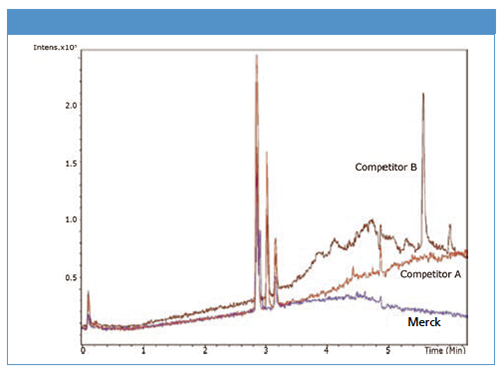
LiChrosolv methanol for UHPLC–MS shows a flat baseline and by far the lowest impurity profile compared to the competition. Both competitor’s high purity UHPLC–MS products A and B show a baseline drift and significant impurity peaks.
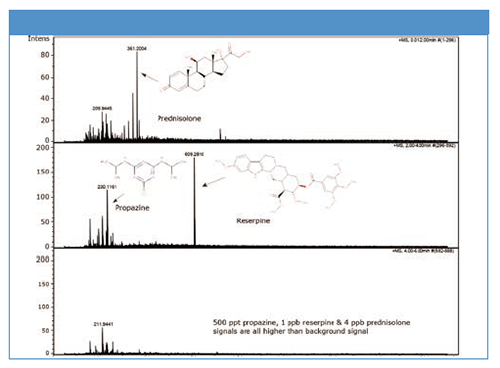
Test 1: UHPLC–MS gradient run with LiChrosolv acetonitrile for UHPLC–MS shows a clear detection and identification of 1 ppb reserpine, 500 ppt propazine, and 4 ppb prednisolone, with very low background interferences.
Test 2: Comparison of LiChrosolv methanol for UHPLC–MS (blue line) with two competitor UHPLC–MS products.
To read the rest of this application please visit SigmaAldrich.com/UHPLC–MS.

Merck
Frankfurter Strasse 250
Darmstadt, 64293, Germany
Website: www.sigmaaldrich.com

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.












