Determination of Choline in Infant Formula and Adult Nutritionals Using Ion Chromatography: AOAC First Action Official Method Performance
Laboratories focusing on ensuring the safety and quality of infant formulas and adult nutritionals require cost effective, rigorous, international consensus methods to address gaps in current approaches. To meet this need, the AOAC International formed its Stakeholder Panel on Infant Formula and Adult Nutritionals (SPIFAN). This article describes the performance of the AOAC Official First Action Method (AOAC Method 2012.20) chosen by SPIFAN, which uses microwave-assisted hydrolysis sample preparation followed by ion chromatography (IC) separation with suppressed conductivity detection for the determination of the SPIFAN priority nutrient choline.
Photo Credit: TEK IMAGE/Getty Images

Laboratories focusing on ensuring the safety and quality of infant formulas and adult nutritionals require cost effective, rigorous, international consensus methods to address gaps in current approaches. To meet this need, the AOAC International formed its Stakeholder Panel on Infant Formula and Adult Nutritionals (SPIFAN). This article describes the performance of the AOAC Official First Action Method (AOAC Method 2012.20) chosen by SPIFAN, which uses microwave-assisted hydrolysis sample preparation followed by ion chromatography (IC) separation with suppressed conductivity detection for the determination of the SPIFAN priority nutrient choline.
Laboratories focusing on ensuring the safety and quality of foods face growing challenges. New infant formulas and adult nutritional dietary supplements continue to be introduced, placing new demands on the methods used to detect and quantify the key components that affect product efficacy and safety. To address gaps in current methodology and to develop new, rigorous, international consensus methods, the AOAC International formed the AOAC Stakeholder Panel on Infant Formula and Adult Nutritionals (SPIFAN), funded through the Infant Nutrition Council of America (INCA).
1
Panels comprised of stakeholder experts from global government, industry, academia, and contract research organizations were formed to establish standard method performance requirements (SMPRs) for each AOAC priority nutrient. AOAC Expert Review Panels (ERPs) were formed to approve First Action
Official Method(s)SM
that subsequently undergo multi-laboratory testing (MLT) prior to becoming Final Action
Official Method(s)SM
. By the end of 2015, 28 SMPRs have been completed and 43 First Action
Official Method(s)SM
adopted.
1
One SPIFAN priority nutrient is choline (Figure 1), a water-soluble quaternary amine essential to methyl metabolism, transmembrane signalling, and brain development. Choline is present in many food matrices, including human breast milk, in free, as well as esterified and bound forms, including acetylcholine, phosphocholine, phosphatidylcholine, glycerophosphocholine, and sphingomyelin. All forms are thought to be available to the body, though not equally.
To foster normal fetal and infant brain development, adequate choline intake is important for mothers during conception and pregnancy, and for infants from birth to at least 12 months of age.
2,3
The adequate intake (AI) for infants aged 0–12 months ranges from 125 to 150 mg/day, while the AI for adult men, pregnant women, and lactating mothers ranges from 450 to 550 mg/day. The AI for adult women who are not pregnant or lactating ranges from ~400 to 425 mg/day.
3,4
Choline deficiency in adults has been associated with both liver and muscle damage.
5
Although the human body produces some choline, consuming a choline-rich diet is necessary to meet dietary needs or to correct deficiencies. Human breast milk is rich in choline, but formulas derived from other sources have lower choline concentrations. For these reasons, infant formulas and certain adult nutritional products are fortified with choline. The most common choline supplement and food additive is lecithin.
Analytical Challenges and Solutions
Prior to SPIFAN’s efforts, AOAC Official Method 999.14, an enzymatic acid hydrolysis/colorimetric method, was the accepted method for determining choline in infant formula and milk. However, the method is limited because it cannot be used to analyze products containing more than 100 mg vitamin C per 100 g solid. The method has also only been validated for the analysis of infant formulas and milk powders. Because choline lacks a chromophore it is not amenable to analysis using high performance liquid chromatography–ultraviolet (HPLC–UV) detection. However, choline is charged and has a charge-to-mass ratio amenable to ion chromatography (IC) with conductivity detection. IC columns specifically designed to enhance separation of amines are readily available. These columns provide improved peak shapes and therefore improved sensitivity. IC methods have the additional advantage of relying on lower cost, safer, and more environmentally friendly aqueous rather then organic solvents. While liquid chromatography tandem mass spectrometry (LC–MS–MS) methods offer another approach, they require more costly technology and are more complicated to operate. This article presents an IC method that uses anion-exchange separation with suppressed conductivity detection for the determination of choline.
AOAC Official First Action Method
AOAC SMPR 2012.013 for the determination of total choline in infant formula and adult/paediatric nutritional formula was finalized on the 29 September 2012.
6
Because IC with suppressed conductivity detection offers a very cost-effective approach for the determination of choline, a single laboratory validation (SLV) study of the method was performed on commercially available samples. At the 2012 AOAC Annual Meeting, the ERP concluded that the results of the IC method met the choline SMPR and it was subsequently approved as AOAC Official First Action Method (AOAC Method 2012.20).
7,8
To further assess the IC method, the ERP requested another SLV study of 17 SPIFAN samples. The second SLV, the focus of this article, provided an opportunity to enhance the method and add microwave-assisted sample preparation to improve sample throughput and recovery.
Samples and Sample Preparation:
Infant formula and adult nutritional samples provided in the SPIFAN SLV Test Materials Kit were analyzed. The samples included a standard reference material (SRM), 11 fortified adult nutritional and infant formulas, and five placebos. Microwave-assisted hydrolysis was used to digest and release bound choline from the powder and ready-to-feed infant formula and adult nutritional samples. The sample was added to the microwave vessel (CEM) and the microwave programme was run to completion. Samples were then filtered and diluted with deionized (DI) water prior to neutralization for IC analysis using a Dionex OnGuard II A Cartridge (Thermo Scientific).
IC Separation and Detection:
Following sample preparation, choline was separated from common cations using a 2 mm x 250 mm, Dionex IonPac CS19 column (Thermo Scientific) and detected by suppressed conductivity.
IC Separation and Detection Parameters:
Columns: 2 mm x 50 mm, Dionex IonPac CG19 Guard (Thermo Scientific); 2 mm x 250 mm, Dionex IonPac CS19 Analytical (Thermo Scientific); eluent gradient: –5 to 13 min 6.4 mM MSA, step to 25 mM MSA at 13 min, 25 mM MSA from 13–17 min; eluent source: Dionex EGC III MSA Eluent Generator Cartridge (Thermo Scientific) with a 5.1 cm x 5.5 cm, 8.4-cm Dionex CR-CTC II Continuously Regenerated Cation Trap column (Thermo Scientific); flow rate: 0.25 mL/min; injection volume: 5 μL; temperature: 30 ËC; autosampler temperature: 10 ËC; detection: suppressed conductivity with Dionex CSRS300 Cation Self-Regenerating Suppressor, 2 mm, Recycle Mode, –5–13 min 5 mA, 13–17 min 19 mA
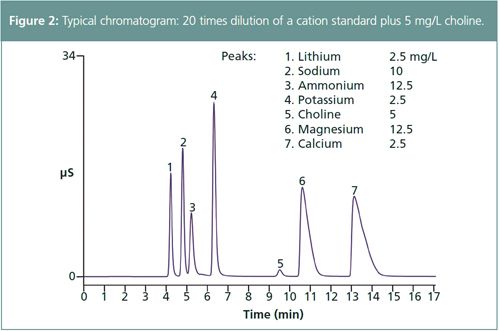
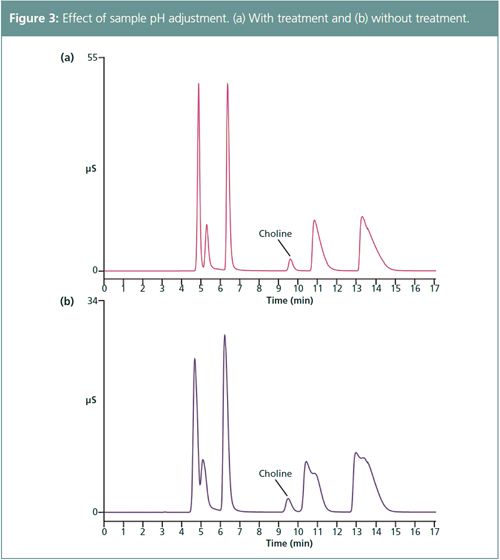
Figure 2 shows a chromatogram of a 5-μL injection for a 20-fold dilution of a Combined Six Cation Standard-II (Thermo Scientific) plus choline. Because the cation exchange sits on the column protonate at low pH, there was a loss in peak efficiency as a result of the sample preparation (Figure 3[b]). Therefore, the sample was treated to adjust the sample to ~pH 6. Figure 3(a) shows the improved peak efficiencies achieved by neutralizing samples.
Results
Validation protocols including linearity, limit of detection (LOD), limit of quantitation (LOQ), precision, accuracy, and recovery were performed according to SPIFAN SLV guidelines.
5
Linearity:
To determine linearity, calibration standards were injected in triplicate over seven concentration levels covering the range specified by the SMPR. The results demonstrated that the detection of choline was linear over the specified range, with a coefficient of determination >0.9999. Errors at each level of the calibration curve were <5%.
LOD and LOQ:
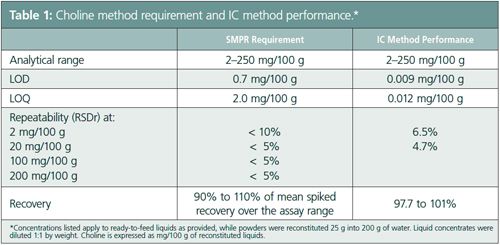
The LOD was first estimated as three times the signal-to-noise ratio (S/N) and the LOQ as 10 times the S/N. Baseline noise for the LOD and LOQ was determined by measuring peak-to-peak noise between 3.4 and 3.9 min over five sample injections. The estimated LOD and LOQ for choline were determined to be 0.003 mg/100 g, and 0.009 mg/100 g, respectively. Spiked blanks containing these amounts were prepared to calculate the final LOD and LOQ. The final LOD was defined as the blank mean plus three standard deviations and the LOQ was defined as the blank mean plus 10 standard deviations. Using this approach, LOD and LOQ were 0.009 mg/100 g and 0.012 mg/100 g, respectively, well below the levels specified in the SMPR as shown in Table 1.
Precision:
Method precision was evaluated by analysis of the 11 fortified test samples and the NIST SRM using multiple instruments. Samples were prepared in duplicate over six days and intermediate and overall reproducibility were calculated. The choline concentration in these samples ranged from 3.00 mg/100 g to 51.5 mg/100 g. Intermediate precision was <6.5% and 4.7% for samples containing choline at 2 mg/100 mg and 20 mg/100 mg, respectively (Table 1). Overall precision was <3.0% for all matrices. To assess precision over the analytical range, selected samples were spiked with 0, 2 mg/100 g, 20 mg/100 g,100 , and 200 mg/100 g choline. Analysis of these samples demonstrated that the method was able to measure choline over the assay range.
Accuracy:
Accuracy (trueness against reference material) was determined by analyzing the NIST SRM in duplicate over six days. The amount of choline found over the six days (n = 12) was 10.2 mg/100 g ± 0.2 mg/100 g with a RSD of 1.8%.
Recovery:
Recovery of choline was determined by spiking duplicate preparations at 50% and 100% of the choline amounts found in the fortified products, directly into the placebo prior to extraction. Good recovery of choline from the five matrices ranging from 92.8% to 101% was obtained. To assess spiked recovery over the analytical range, placebos were spiked with 2.5, 20, 100, and 200 mg/100 g choline. Recoveries ranged from 97.7% to 101% (Table 1).
Conclusion
With the constant introduction of new infant formulas and adult nutritional supplements, robust consensus methods are needed to detect and quantify important components such as choline. Microwave-assisted hydrolysis followed by IC separation with conductivity detection enabled robust, accurate, and sensitive determination of choline in SPIFAN SLV test samples per the SMPR requirements. Microwave-assisted digestion effectively hydrolyzed bound choline to its free form, speeding up sample preparation and increasing sample throughput compared to previous approaches. As the SPIFAN moves forward, First Action Methods will undergo multi-laboratory testing. After testing is completed, the AOAC will continue to work with the ERPs to facilitate the review and recommendation of methods for Final Action
Official Method(s)SM
status.
References
1. Stakeholder Panel on Infant Formula and Adult Nutritionals (SPIFAN). AOAC International: http://stakeholder.aoac.org/SPIFAN/aoac.html (accessed 7 January 2016). 2. G.M. Shaw, S.L. Carmichael, W. Yang, S. Selvin, and D.M. Schaffer,
American Journal of Epidemiology
160
(2), 102–9 (2004). 3. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academies Press (US): Washington, DC. USA: http://www.ncbi.nlm.nih.gov/books/NBK114310/pdf/TOC.pdf (accessed 3 February 2014). 4. Volume 2, Section 107.100 Nutrient Specifications. CFR-Code of Federal Regulations Title 21; U.S. Food and Drug Administration: Silver Spring, MD, 2011: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=107.100 (accessed 4 January 2016). 5. L.M. Sanders and S.H. Zeisel,
Nutr. Today
42
(4), 181–186 (2007). 6. AOAC SMPR 2012.013 Standard Method Performance Requirements for Total Choline in Infant Formula and Adult Pediatric Nutritional Formula Reference,
J. AOAC Int.
96
, 492 (2013). 7. K. Oates, L. Chen, B. De Borba, D. Mohindra, and J. Rohrer,
J. AOAC Int.
96
(7), 1400–1406 (2013). 8. AOAC Official Method 2012.20. Choline in Infant Formula and Adult Nutritionals. Ion Chromatography and Suppressed Conductivity. First Action 2012: http://stakeholder.aoac.org/SPIFAN/2012.20.pdf (accessed 7 January 2016).
Jeffrey Rohrer
is director of Applications Development, Dionex Products, for Thermo Fisher Scientific. He received his PhD in chemistry from the University of Delaware, Delaware, USA, and did post-doctoral research in the Department of Biochemistry at North Carolina State University, North Carolina, USA.













