Extraction and Quantification of Mycotoxins in Peanut Butter Using SFE–SFC–MS
The Column
Aflatoxins are poisonous and carcinogenic chemicals that occur in nature in soil, hay, and grains. When contaminated food is processed, aflatoxins can enter the general food supply chain and can be dangerous for humans and animals. Time- and cost-efficient analytical methods can ensure food safety. Compared to conventional manual methods the use of “supercritical” CO2 for supercritical fluid extraction (SFE) as well as chromatographic separation (supercritical fluid chromatography [SFC]) of aflatoxins offers a more efficient process while reducing solvent consumption.
Photo Credit: Image Studios/Getty Images

Aflatoxins are poisonous and carcinogenic chemicals that occur in nature in soil, hay, and grains. When contaminated food is processed, aflatoxins can enter the general food supply chain and can be dangerous for humans and animals. Time- and cost-efficient analytical methods can ensure food safety. Compared to conventional manual methods the use of “supercritical” CO2 for supercritical fluid extraction (SFE) as well as chromatographic separation (supercritical fluid chromatography [SFC]) of aflatoxins offers a more efficient process while reducing solvent consumption.
Aflatoxins are mycotoxins produced by fungal molds of the
Aspergillus
species, which prefer a warm and damp climate. Aflatoxins can be produced by fungal infestation during or after harvest of peanuts and can therefore end up in processed products such as peanut butter or satay sauce. In addition to being acutely toxic, they are also known to be carcinogenic. Aflatoxin B1 is the most common type of aflatoxin present in food products, and it is also one of the most genotoxic and carcinogenic species.
1
To ensure food safety, manufacturers of food and beverages have to strictly manage risks from such contaminants, and sensitive methods to assay mycotoxins in complex matrices are essential. A conventional, manual method for sample extraction of aflatoxins from peanut butter is time consuming; it involves weighing of the sample, adding a suitable solvent, and homogenizing for about 30 min, followed by filtering. The sample has to be defatted at least twice by extracting with hexane and then again with chloroform. Only after evaporation of the chloroform can the residue be dissolved in a suitable solvent for clean up by solid-phase extraction (SPE) before analysis using a chromatographic technique.
2
The use of “supercritical” CO
2
for extraction as well as chromatographic separation can offer a more efficient extraction process while reducing solvent consumption and the risk of losses or error of measurement during sample preparation. Furthermore, extraction selectivity can be carefully controlled by adjusting the solvating power of the CO
2
through changes in pressure and temperature, making it faster, more efficient, and more versatile than liquid and SPE.
3
Supercritical CO
2
is a fluid state of carbon dioxide where it is held at or above its critical temperature (31.1 °C) and critical pressure (73.8 bar). In this supercritical state, fluid density is strongly affected by temperature and pressure, and its physical and thermal properties are between those of the liquid and the gaseous phase.
4
Compressibility and diffusion coefficient of the supercritical solvent are higher, while viscosity is decreased compared to a pure liquid, making it better suited to permeation of penetrable solids.
Method
In the on-line SFE-SFC approach, the peanut butter sample was homogenized with an absorbent powder and placed in the extraction vessel in the SFE unit (both Shimadzu). Supercritical CO
2
was then introduced into the vessel, where extraction conditions were optimized with respect to pressure and temperature. After extraction in the SFE unit, the sample-containing CO
2
was introduced in the SFC flow line for chromatographic analysis by supercritical fluid chromatography (SFC).
SFE Conditions:
Extraction vessel: 5 mL; vessel temperature: 40 °C; flow rate: 5.0 mL/min; back pressure regulator (BPR) pressure: A: 150 bar (to column), B: 150 bar (to waste); static extraction: B. concentration 30% (0–1 min), 0% (1.01– 2 min); dynamic extraction: B. concentration 0% (2–4 min).
SFC Conditions:
Column: 4.6 mm x 250 mm, 5-μm Cosmosil π-NAP (Nacalai Tesque); mobile phase: A: CO2, B: methanol; flow rate: 3.0 mL/min; gradient programme: B. concentration 0% (0–2 min) – 50% (16 min); make-up solution: 10 mmol/L ammonium acetate in methanol; make-up flow rate: 0.3 mL/min; column temperature: 40 °C; BPR pressure: A: 150 bar (to column); B: 150 bar (to waste); BPR temperature: A: 50 °C, B: 50 °C.
Mass Spectrometry (MS) Detection:
System: LCMS-8050 triple quadrupole spectrometer (Shimadzu); probe voltage: 4 kV (ESI-positive); DL temperature: 250 °C; block heater temperature: 400 °C; interface temperature: 300 °C; nebulizing gas flow: 3 L/min; drying gas flow: 10 L/min; heating gas flow: 10 L/min; MRM transition: aflatoxin B1 (positive 313.10 > 241.10, 313.10 > 269.10, 313.10 > 285.20), aflatoxin B2 (positive 315.00 > 259.10, 315.00 > 287.15, 315.00 > 243.15).
Results
Determination of Linearity:
Linearity of the on-line SFE–SFC–MS analysis of aflatoxins B1 and B2 was established by creating a calibration curve using the standard addition method. A 2 µL sample of 0.1, 1, and 10 ppm aflatoxin standard solution was added to the mixture of peanut butter and absorbent in the extraction vessel, creating spiked samples of 0.2, 2, and 20 ng/g aflatoxin concentration. Partial chromatograms and linearity curves are displayed in Figure 1.
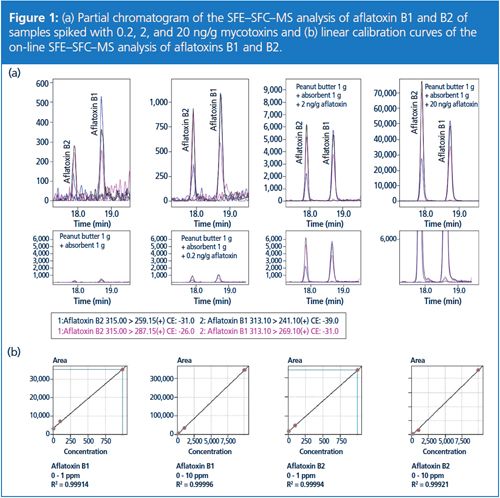
Determination of Repeatability:
Repeatability of the on-line SFE–SFC–MS analysis of aflatoxins B1 and B2 was established by performing six successive SFE–SFC–MS analyses of the mixture of peanut butter and absorbent spiked with 2 µL of 1 ppm aflatoxin standard solution. Relative standard deviations (RSD) were 0.03% and 0.04%, respectively. Peak area RSD was 12% for aflatoxin B1 and 13% for aflatoxin B2.
Conclusion
The online SFE–SFC–MS approach was successfully used to extract and analyze aflatoxin B1 and B2 from spiked peanut butter samples in only a fraction of the time that a conventional liquid extraction followed by SPE clean-up sample preparation method would take. The procedure was shown to be linear in a concentration range of 0–10 ppm with good reproducibility. The use of supercritical CO
2
in industrial processes involving food or pharmaceuticals eliminates the possible presence of residual solvents, which is always a concern when a "typical" organic solvent is used. An additional benefit is the elimination of disposal costs associated with otherwise large volumes of organic eluents. SFE and SFC are faster, more efficient, less costly, and more environmentally friendly than other previously mentioned methods that require the use of large amounts of toxic organic solvents.
References
[1] European Food Safety Authority: http://www.efsa.europa.eu/de/topics/topic/aflatoxins [2] Application Note on Extraction of Aflatoxins (B1, B2, G1, and G2) from Corn, Peanuts and Peanut Butter 2012 Avantor Performance Materials, Inc. [3] T. Letzel and S. Bieber,
Analytik News
8
(2015). [4] L.T. Taylor,
Anal. Chem.
82
(12), 4925–493 (2010).
Gesa Johanna Schad
graduated with a diploma in chemical engineering from the Technical University, NTA in Isny, Germany, in 2004 and as a Master of Science in pharmaceutical analysis from the University of Strathclyde in Glasgow, UK, in 2005. Since 2013, she has worked in the analytical business unit of Shimadzu Europa in Duisburg, Germany, and since 2015 as HPLC product manager.
Kenichiro Tanaka
graduated with a bachelor's degree in engineering in 2004 and a master's degree in engineering in 2006, both from Kyoto University in Japan. He joined Shimadzu Corporation in Japan in 2006 and worked as an HPLC applications chemist. Since 2012 he has worked as an HPLC applications chemist at Shimadzu Scientific Instruments, Inc.
Tairo Ogura
graduated from the school of agriculture of Meiji University in Japan in 2002, and gained a master’s degree in science from the integrated science cooperative graduate school of Yokohama City University in Japan in 2004. After joining Shimadzu Corporation in 2004 he worked as an LC–MS applications scientist in Japan. He completed a doctorate in engineering at Osaka University in Japan in 2015 and has worked at Shimadzu Scientific Instruments, Inc. in the US as an applications manager since then.

Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
Using GC-MS to Measure Improvement Efforts to TNT-Contaminated Soil
April 29th 2025Researchers developing a plant microbial consortium that can repair in-situ high concentration TNT (1434 mg/kg) contaminated soil, as well as overcome the limitations of previous studies that only focused on simulated pollution, used untargeted metabolone gas chromatography-mass spectrometry (GC-MS) to measure their success.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.
Potential Obstacles in Chromatographic Analyses Distinguishing Marijuana from Hemp
April 28th 2025LCGC International's April series for National Cannabis Awareness Month concludes with a discussion with Walter B. Wilson from the National Institute of Standard and Technology’s (NIST’s) Chemical Sciences Division regarding recent research his team conducted investigating chromatographic interferences that can potentially inflate the levels of Δ9-THC in Cannabis sativa plant samples, and possible solutions to avoid this problem.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












