Desorption and Ionization Mass Spectrometry in Research Laboratories
LCGC North America
The past decade has brought exponential growth in the number of mass spectrometry (MS) ionization techniques based on desorption and ionization (DI) processes. Here, the three key applications for DI are discussed: rapid, in situ screening; direct analysis of extracted samples or of planar chromatography spots; and scanning samples along x and y axes.
The past decade has brought exponential growth in the number of mass spectrometry (MS) ionization techniques based on desorption and ionization (DI) processes. Here, the three key applications for DI are discussed: rapid, in situ screening; direct analysis of extracted samples or planar chromatography spots; and scanning samples along x and y axes.
The past decade has brought exponential growth in the number of mass spectrometry (MS) ionization techniques based on desorption and ionization (DI) processes (Figure 1). These techniques are initiated by desorption of molecules from a sample followed by ionization and analysis by MS. There are several methods of desorption in use by the community that fall into three main categories. Desorption can occur by means of a focused beam or spray (for example, laser, ions, or charged droplets of solvents); desorption can be initiated by focused acoustic and thermal energy; or it can arise as a result of any combination of the above. Additionally, with matrix assistance desorption can occur by exposing samples to vacuum without any external energy input (1).
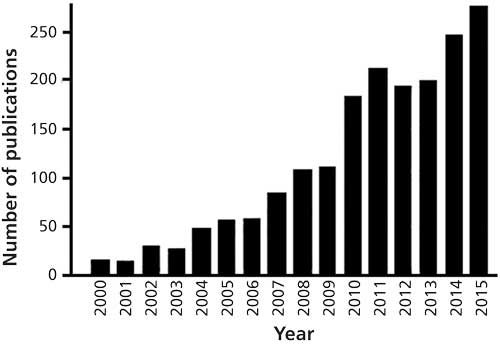
Figure 1: The number of articles published per year, from 2000 to 2015, on the topic of desorption ionization and imaging. (Based on the Web of Science database; Thomson Reuters, New York, New York.)
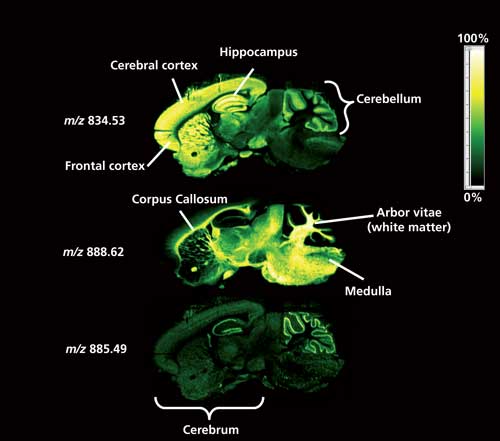
Figure 2: A high spatial resolution DESI-MS image of lipid species present in a sagittal mouse brain section is able to identify ions located in distinct regions of the brain. (Image courtesy of Emrys Jones, Waters Corporation.)
Currently, there are more than 25 described DI techniques, each with their own advantages and disadvantages for specific classes of samples and applications. Some, such as matrix-assisted laser desorption–ionization (MALDI), secondary ion MS (SIMS), and nanostructure-initiator mass spectrometry (NIMS), operate under vacuum. Others, known as ambient ionization MS (AIMS) techniques, operate at atmospheric pressure, allowing for minimal sample preparation before analysis. AIMS techniques include desorption electrospray ionization (DESI), rapid evaporative ionization mass spectrometry (REIMS), direct analysis in real time (DART), and laser ablation electrospray ionization (LAESI).
The three key applications for DI are rapid, in situ screening; direct analysis of extracted samples or planar chromatography spots; and scanning samples along x and y axes.
Rapid In Situ Screening of Cells, Biological Fluids, and Tissues
DI-MS can be used for real-time, rapid, in situ screening of metabolites and lipids within cells, biological fluids, and tissues. Also, it allows for direct analysis of bacterial colonies, dried blood spots, and many other sample types (2–6).
One of the most exciting AIMS techniques described is REIMS. In the most widely used modality of REIMS, a rapid evaporative thermal process generates aerosol on a sample that is transported inside the mass spectrometer via a flexible tube. The aerosols are ionized by impact with a heated coil and ions are detected by a quadrupole time-of-flight (QTOF) mass spectrometer. Within a few seconds of producing mass spectra, results are processed with informatics tools and displayed in a format understood by nonexpert users to assist in their analytical decision. One of the main advantages of this DI-MS approach is the ability to generate molecular fingerprints in real time that can potentially be used to discriminate among phenotypes (for example, distinguishing healthy from diseased tissue and bacterium A from bacterium B). Significantly, the technique’s ability to get real-time molecular information from any surface using a sampling device tethered to flexible tubing removes the need for any sample preparation (7). Thus, DI-MS offers a practical and convenient opportunity for the development of portable devices for diagnostics applications.
Direct Analysis of Extracted Samples or Planar Chromatography Spots
DI-MS can be used for direct analysis of extracted samples or planar chromatography spots, such as those derived by paper chromatography, thin-layer chromatography (TLC) or high performance TLC (HPTLC), and even silicon nanowires. Processed tissues, like lipid extracts, can be analyzed without the necessity of special surfaces that favor the ionization process. Robotic systems for in situ lipid extraction (by either solvent or solid-phase extraction) and deposition have been shown to increase the dynamic range of detection. Planar chromatography is a convenient method for separating lipid species. In the past, unknown lipids were scraped from paper or a TLC or HPTLC plate, eluted into a tube, and transferred into the mass spectrometer. Today, planar chromatography, with great effect and small effort, can be interfaced with DI-MS to automatically analyze lipids on paper or plates.
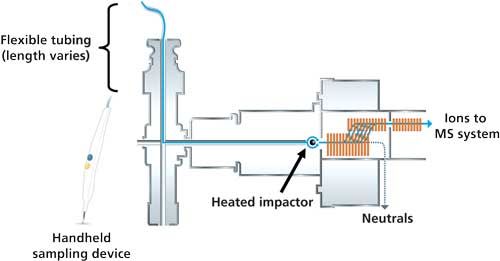
Figure 3: A typical workflow of REIMS is shown. A handheld sampling device tethered to flexible tubing is used in desorption of sample. The sample plume is transported to mass spectrometer and ionized by a heated impactor. The molecular profile of the sample is then compared to a database, and the sample is identified in real time. (Image courtesy of Waters Corporation.)
Scanning Tissues and Cells Along x and y Axes
DI-MS can be used to scan through tissues and cells, along the x and y axes, to create topographic maps of molecular composition. This technique is referred to as MS imaging (MSI) (8). For example, little is known about the precise distribution of molecular species in distinct anatomical features of many animal and plant tissues. Laser-capture microdissection and differential centrifugation can isolate fine structures of tissues. However, in the preparation for MS analysis those samples generally require processing and extraction, which can chemically alter the molecules under study. MSI allows us to detect molecules in their natural location by scanning through tissues and generating topographical maps of the molecular distribution on the surface.
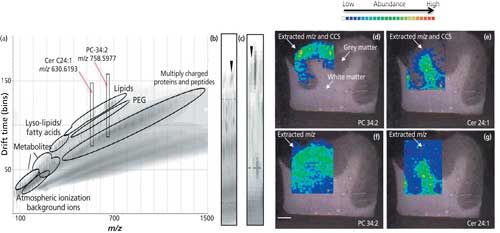
Figure 4: The use of ion mobility separation and collision cross section (CCS) in ambient ionization mass spectrometry is shown. Lipids of interest in brain sections are imaged from complex LAESI mass spectra composed of metabolites, lipids, and proteins. MS images can be overlaid onto more conventional imaging modalities. (Adapted with permission from reference 14.)
Some of the first DI technologies to generate molecular maps from biological samples were SIMS and MALDI, which can resolve features as small as submicrometer and tens of micrometers, respectively. Impressive as they are, however, those techniques do have some limitations. SIMS suffers from analyte fragmentation, the unintended effect of high-energy ion beams, which can be overcome somewhat by the use of polyatomic ionic particles such as C60+ clusters. In MALDI, the small organic molecules that serve as a matrix can produce significant background noise that can overwhelm the signal, complicating the analysis of low-mass-range molecules.
Several new matrix-free DI techniques have recently been introduced for MS imaging that counter some of these limitations by allowing direct analysis of samples. These include DESI, LAESI, NIMS, and nanophotonic laser desorption ionization from silicon nanopost arrays (9)-all of which offer the advantages of low chemical noise and high sensitivity in the low-mass range. In particular, DESI is becoming widely used for the molecular distribution profiling of metabolite and lipid species from a wide range of tissue sections and microbial colonies. As examples, DESI has been utilized to investigate potential differences between gliomas from biopsies of brain tissues (10), the distribution of natural products within microbial colony (11), and follicular transport of the drug lidocaine into the deep skin layers (12). In DESI, ions are generated at ambient conditions by desorption and ionization of analytes from the sample surface by pneumatically assisted electrospray. The ions produced are very similar to those obtained by electrospray ionization.
DI-MS Coupled with Ion Mobility
Two significant challenges in DI-MS experiments are accurate analyte localization, which may be affected by the presence of confounding isobaric species, and analyte identification, which relies, mostly, on accurate mass alone. That’s where combining ion mobility (IM) with traditional DI-MS and MS imaging approaches can help (13).
IM is a technique that enables separating gas-phase ions according to their physical size, shape, and charge. IM separations occur on a timescale of milliseconds, yielding a peak capacity production rate for IM-MS of 106–108/s, which makes IM ideal for coupling with fast-scanning TOF-like detectors. Because IM disperses mass signals across another dimension of information (mobility drift time), different classes of molecules can be separated by ion mobility post DI, according to their chemical structures (14). Therefore, combining IM with DI-MS benefits from an enhanced peak capacity and signal-to-noise ratio, compared with the conventional, single dimension of DI-MS analysis.
In addition, from the characteristic mobility drift time, it is possible to calculate the rotationally averaged collision cross section (CCS), which represents the effective area for the interaction between an individual ion and the neutral gas through which it travels. Obtaining the CCS values for an analyte and adding them to accurate mass measurements provides complementary information about an analyte (14). The characteristic CCS value associated with each desorbed analyte ion can be identified against a database. In this way, IM-derived CCS information enhances the specificity of analyte identification and data interpretation in DI-MS experiments (15).
Conclusions
In the last decade, we have observed a series of technological advancements in the field of DI-MS approaches. These advances are revolutionizing research in environmental sciences, food sciences, drug discovery, and disease diagnostics. In addition to MALDI-MS, several AIMS technologies have been commercialized, notable among them are DESI, REIMS, LAESI, and DART. The thorough integration of such sources with commercial mass spectrometers and software will encourage more researchers to adopt AIMS for their applications, potentially transforming the way that many analyses are currently conducted.
References
- S. Trimpin, J. Am. Soc. Mass Spectrom. 27, 4 (2016).
- J.M. Wiseman, D.R. Ifa, Q. Song, and R.G. Cooks, Angew. Chem., Int. Ed. 45, 7188 (2006).
- C.Y. Pierce, J.R. Barr, R.B. Cody, R.F. Massung, A.R. Woolfitt, H. Moura, H.A. Thompson, and F.M. Fernandez, Chem. Commun. 8, 807–809 (2007).
- J. Shiea, M.Z. Huang, H.J. HSu, C.Y. Lee, C.H. Yuan, I. Beech, and J. Sunner, Rapid Commun. Mass Spectrom. 19, 3701 (2005).
- B. Shrestha, P. Nemes, J. Nazarian, Y. Hathout, E.P. Hoffman, and A. Vertes, Analyst135, 751 (2010).
- T.R. Northen, O. Yanes, M.T. Northen, D. Marrinucci, W. Uritboonthai, J. Apon, S.L. Golledge, A. Nordström, and G. Siuzdak, Nature449, 1033 (2007).
- K.C. Schäfer, J. Dénes, K. Albrecht, T. Szaniszló, J. Balog, R. Skoumal, M. Katona, M. Tóth, L. Balogh, and Z. Takáts, Angew. Chem., Int. Ed.48, 8240 (2009).
- M. Stoeckli, P. Chaurand, D.E. Hallahan, and R.M. Caprioli, Nat. Med.7, 493 (2001).
- B.N. Walker, J.A. Stolee, and A. Vertes, Anal. Chem.84, 7756 (2012).
- L.S. Eberlin, I. Norton, A.L. Dill, A.J. Golby, K.L. Ligon, S. Santagata, R.G. Cooks, and N.Y. Agar, Cancer Res.72, 645 (2012).
- E. Esquenazi, Y.-L. Yang, J. Watrous, W.H. Gerwick, and P.C. Dorrestein, Nat. Prod. Rep.26, 1521 (2009).
- J. D’Alvise, R. Mortensen, S.H. Hansen, and C. Janfelt, Anal. Bioanal. Chem. 406, 3735 (2014).
- J.A. McLean, W.B. Ridenour, and R.M. Caprioli, J. Mass Spectrom. 42, 1099 (2007).
- G. Paglia, J.P. Williams, L. Menikarachchi, J.W. Thompson, R. Tyldesley-Worster, S. Halldórsson, O. Rolfsson, A. Moseley, D. Grant, J. Langridge, B.O. Palsson, and G. Astarita, Anal. Chem.86, 3985 (2014).
- M. Kliman, J.C. May, and J.A. McLean, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids1811, 935 (2011).

Bindesh Shrestha is a senior application support scientist at Waters Corporation. He has coauthored more than two dozen peer-reviewed articles in the field of ambient ionization mass spectrometry (AIMS) spanning the last decade. He has extensive experience in developing various AIMS ion sources, such as an atmospheric pressure matrix-assisted laser desorption/ionization (MALDI) and laser ablation electrospray ionization (LAESI), to spatially map biomolecules in tissues and single cells. Dr. Shrestha’s publications have been featured multiple times on the covers of leading journals, such as Angewandte Chemie, Analytical Chemistry, Analyst, Analytica Chimica Acta, and Chemical Communications.

Giuseppe Astarita is a principal scientist at Waters Corporation (Milford, Massachusetts) and serves as adjunct professor at Georgetown University (Washington, DC). Dr. Astarita’s research interests include lipid mass spectrometry and metabolomics, as applied to translational medicine and biomarker discovery. He is applying the latest technologies in the study of health and disease as well as food and nutrition.

Kate Yu “MS-The Practical Art” Editor Kate Yu joined Waters in Milford, Massachusetts, in 1998. She has a wealth of experience in applying LC–MS technologies to various application fields such as metabolite identification, metabolomics, quantitative bioanalysis, natural products, and environmental applications.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)