Separation of Amino Acid Enantiomers Using Capillary Electrophoresis with a New Chiral Ligand
LCGC North America
A chiral ionic liquid, namely 1-ethyl-3-methyl imidazole L-tartrate ([EMIM][L-Tar]), was applied as a new chiral ligand for the enantioseparation of tryptophan, tyrosine, and phenylalanine enantiomers by chiral ligand exchange CE. To validate the unique behavior of [EMIM][L-Tar], the performance of L-tartaric acid and 1-ethyl-3-methyl imidazole L-proline as chiral ligands was investigated to make a comparison with [EMIM][L-Tar]. Then the separation mechanism was further discussed. It was proven that [EMIM][L-Tar] was a good chiral ligand and would have good application prospects in separation science.
A chiral ionic liquid, namely 1-ethyl-3-methyl imidazole L-tartrate ([EMIM][L-Tar]), was applied as a new chiral ligand for the separation of tryptophan, tyrosine, and phenylalanine enantiomers by chiral ligand exchange capillary electrophoresis (CE). To validate the unique behavior of [EMIM][L-Tar], the performance of L-tartaric acid and 1-ethyl-3-methyl imidazole L-proline as chiral ligands was investigated to make a comparison with [EMIM][L-Tar]. Then the separation mechanism was further discussed. It was proven that [EMIM][L-Tar] was a good chiral ligand and would have good application prospects in separation science.
Chirality plays a unique role in the origin and evolution of life, hence enantioseparations of chiral compounds have drawn considerable attention in the past few decades. Separation techniques for chiral compounds include chromatographic and electromigration techniques, physical and chemical resolutions, and membrane and enzymic techniques-among which chromatographic and electromigration techniques have shown the greatest superiority (1). Compared to other techniques, capillary electrophoresis (CE) has several advantages: high separation efficiency; low consumption of samples, solvents, and chiral selectors; and high flexibility in choosing and changing types of chiral selectors (2).
There are many kinds of chiral selectors used in CE, such as cyclodextrins (3,4), chiral crown ethers (5), macrocyclic antibiotics (6), polysaccharides (7), proteins (8), chiral ionic liquids (9), chiral metal complexes (10), and so on. The separation mode using such chiral metal complexes in CE is called chiral ligand exchange CE (CLE-CE), which is mainly applied for the enantioseparations of amino acids, hydroxy acids, and dipeptides (11–13). CLE-CE uses the exchange interactions between enantiomers and complexes formed by chiral ligands and central ions to achieve the enantioseparations. Therefore, the selection of chiral ligands is of great importance. However, chiral ligands used in CLE-CE are limited within amino acids, amino alcohols, and α-hydroxy acids (14–16) because of the need for high optical purity and easy coordination with transition metal that restricts the development of CLE-CE. Therefore, it is necessary to explore new chiral ligands to widen the range of application.
Ionic liquids (ILs), also known as liquid organic, molten, or fused salts, are a class of nonmolecular ionic solvents with low melting points (17). ILs have many unique properties, such as low vapor pressure, wide operating temperature range, and good thermal and chemical stabilities (18–21). If chirality is introduced into the structure of ILs, then chiral ILs are obtained. Chiral ILs, as the chiral solvents or chiral inducers, have been successfully applied in asymmetric synthesis (22,23), gas chromatography (GC) (24), liquid chromatography (LC) (25,26), and CE (27–30). At present, there are some chiral ILs used as chiral ligands in CLE-CE, such as 1-alkyl-3-methyl imidazole-L-proline (27), chiral ILs with L-ornithine as the anion (28), chiral ILs with L-proline as the cation (29), and chiral ILs with L-lysine as the anion (30).
In this work, a chiral ionic liquid, namely 1-ethyl-3-methyl imidazole-L-tartrate ([EMIM][L-Tar]), was used as the chiral ligand for the enantioseparations of tryptophan (Trp) enantiomers, tyrosine (Tyr) enantiomers, and phenylalanine (Phe) enantiomers. Factors that influenced chiral resolutions were investigated in detail. Compared to L-tartaric acid (L-Tar) and 1-ethyl-3-methyl imidazole L-proline ([EMIM][L-Pro]) as chiral ligands, [EMIM][L-Tar] exhibited good enantiomeric recognition and separation potency. Furthermore, the mechanism of [EMIM][L-Tar] as the chiral ligand in CLE-CE was explored.
Experimental
Chemicals
[EMIM][L-Tar] (99%), [EMIM][L-Pro] (99%), and 1-ethyl-3-methyl imidazole acetate (EMIM-Ace, 99%) used in this work were purchased from Shanghai Chengjie Chemical. D-Trp, L-Trp, D-Tyr, L-Tyr, D-Phe, and L-Phe of 99% were obtained from Aladdin-reagent. Sodium dodecyl sulfate (SDS, 99%) was from Alfa Aesar. L-Tar (99%) was from Sinopharm Chemical Reagent Co., Ltd. Nickel sulfate, copper chloride, and other reagents were all of analytical grade and were purchased from Tianjin Fuchen Chemical Reagents Factory. Double distilled water was used throughout all of the experiments.
CE and Procedures
A CAPEL 105 electrophoresis system with ultraviolet (UV) detection (200 nm) (Lumex) was used. Electrophoresis was performed in untreated fused-silica capillaries (Ruifeng) of 61 cm length (effective length, 52 cm) and 50 μm i.d. (365 μm o.d.). A PHS-3C pH meter (Leici) was used to measure the pH of solutions. Before use, the capillary was washed with 0.1 M sodium hydroxide, distilled water, and running buffers for 5 min. The samples were introduced by a 35 mbar pressure for 30 s. The capillary was held at 20 °C and the applied voltage was 25 kV.
Three pairs of enantiomers were prepared individually. D-Trp together with L-Trp, and D-Phe with L-Phe were dissolved in water at a concentration of 1 mg/mL. While D-Tyr and L-Tyr were first dissolved with 0.1 M sodium hydroxide, they were then diluted with water to a concentration of 1 mg/mL. The standard solutions were diluted to the desired concentration with water before use. Running buffers were freshly prepared by dissolving chiral ligands, metal salts, and other additives in buffer solutions, and then adjusting to a desired pH by adding 1 M sodium hydroxide or 1 M hydrochloric acid. Before use, all solutions were degassed by sonication.
Calculation
The enantioseparation resolution (Rs) of enantiomers was calculated with equation 1:
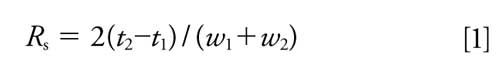
where t1 and t2 are the migration times, w1 and w2 are the peak widths (31).
The electroosmotic flow (EOF) mobility of the enantiomer (μeof) was calculated with equation 2:
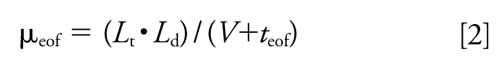
where Lt is the total capillary length, Ld is the effective capillary length, V is the applied voltage, and teof is the migration time of acetone (31).
The effective electrophoretic mobility of the enantiomer (μep) was calculated with equation 3:
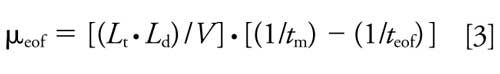
where tm is the migration time of the enantiomer (32).
Results and Discussion
Optimization of Enantioseparation Conditions
Choice of Background Electrolyte
Common buffer solutions, such as borate, phosphate, and citrate, were investigated for the enantioseparations. Borate was finally chosen because of its higher sensitivities and better resolution of enantiomers.
Choice of Central Ion
Four kinds of common central ions (Cu2+, Ni2+, Zn2+, and Co2+) were studied for enantioseparations of Trp enantiomers (as shown in Table I). Using [EMIM][L-Tar] under alkaline conditions, chiral complexes with Ni2+ showed the best enantioseparation ability, while partial enantioseparations were obtained by chiral complexes with Zn2+ and Co2+, and no enantioseparation was found with Cu2+. Furthermore, metallic salts with different anions also exhibited different enantioseparation abilities, for example, nickel sulfate provided much better enantioseparations than nickel chloride and nickel acetate.
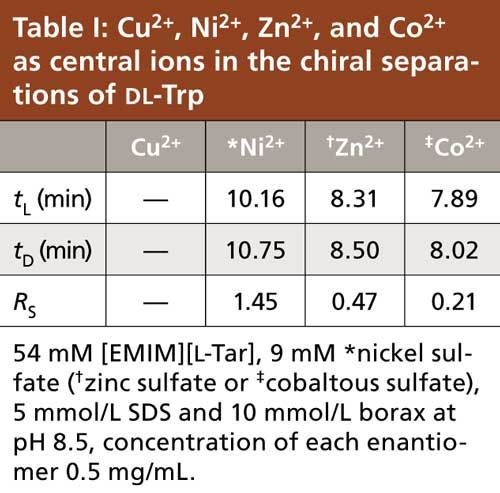
Effect of the Ratio and Concentration
Both the molar ratio of ligand to central ion and the concentration of ligand and central ion have great influences on the enantioseparation. Figure 1 shows the effects of the ratio of [EMIM][L-Tar] to Ni2+ on the enantioseparation resolution (Rs) and migration time. As displayed in Figure 1, both the migration time and Rs increased first and then decreased. The best ratio of [EMIM][L-Tar] to Ni2+ was 6:1. This result indicated that the complexation ability of [EMIM][L-Tar] to Ni2+ was weak, hence [EMIM][L-Tar] needed a higher concentration to achieve the formation of the chiral complexes so as to promote the ligand exchange between the chiral complexes and test enantiomers.
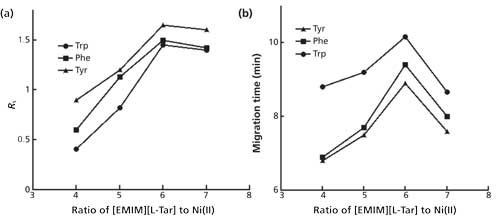
Figure 1: Effects of the ratio of [EMIM][L-Tar] to Ni2+ on Rs and migration time. Running buffer: 5 mmol/L SDS, 10 mmol/L borax, 9 mM nickel sulfate and desired concentration of [EMIM][L-Tar] at pH 8.5, concentration of each enantiomer 0.5 mg/mL.
The influences of [EMIM][L-Tar] at different concentrations were further investigated (Figure 2). It was found that Rs became larger when the increase of the concentration ranged from 36 to 54 mM. However, when the concentration reached up to 60 mM, Rs declined with poor sensitivities and shorter migration time. As a result, the optimum concentration of [EMIM][L-Tar] and Ni2+ was 54 mM and 9 mM.
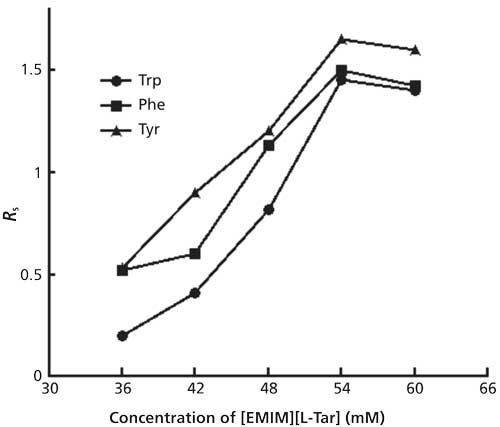
Figure 2: Effects of [EMIM][L-Tar] and Ni2+ at different concentration on Rs. Running buffer: the ratio of [EMIM][L-Tar] to Ni2+ was 6:1. Other conditions were the same as in Figure 1.
Effect of pH
The pH also plays an important role in enantioseparation. It was found that using [EMIM][L-Tar]/Ni2+ under alkaline conditions (pH 7.5–9.5), the migration time decreased while Rs increased with the increase of the pH value. Finally, 8.5 was chosen as the optimum since it was close to the initial pH of the running buffer.
SDS was added to improve the enantioseparations in [EMIM][L-Tar] systems. Under optimal conditions, the well-separated electropherograms of three model analytes using [EMIM][L-Tar] as the chiral ligand are shown in Figure 3.
Figure 3: Electropherograms of three model analytes using [EMIM][L-Tar]. Running buffer: 54 mM [EMIM][L-Tar], 9 mM nickel sulfate, 5 mM SDS, 10 mM borax at pH 8.5. The concentration of each enantiomer was 0.2 mg/mL. First peaks were L-enantiomers, and second peaks were D-enantiomers (a) = Tyr; (b) = Phe; and (c) = Trp.
Comparative Study
To validate the unique behavior of [EMIM][L-Tar], the performance of L-Tar and [EMIM][L-Pro] as chiral ligands was investigated to make a comparison with [EMIM][L-Tar]. Taking Trp enatiomers as an example, under the optimized conditions, partial enantioseparation of Trp enantiomers was obtained when using L-Tar alone, while better enantioseparations were achieved by adding EMIM-Ace, which prolonged the migration time to more than 30 min (Figures 4a and 4b). [EMIM][L-Pro] was also used as the chiral ligand (Figure 4c), and the migration time in this system was more than 20 min. As shown in Figure 3c, the migration time of Trp enantiomers in [EMIM][L-Tar] was less than 13 min, which was much shorter than in other systems. Therefore, compared to the chiral ligands mentioned above, [EMIM][L-Tar] exhibited good enantiomeric recognition and separation potency, and could be used as the effective chiral ligand in CLE-CE.
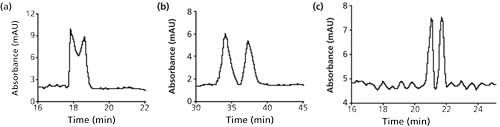
Figure 4: Comparative separations of Trp enantiomers. Running buffer: (a) 20 mM L-Tar, 5 mM cupric chloride at pH 5.0; (b) 20 mM L-Tar, 5 mM cupric chloride, 5 mM EMIM-Ace at pH 5.0; and (c) 40 mM [EMIM][L-Pro], 20 mM cupric chloride at pH 3.5. Other conditions were the same as in Figure 3.
Separation Mechanism
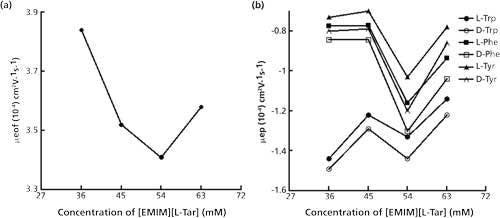
The enantioseparation in CLE-CE is based on the differences in the stability of complexes formed by different enantiomers with chiral ligands and central ions. The different stability causes different migration time, so that the enantiomers could be separated. And the migration time of enantiomers is mainly influenced by μeof and μep. Hence, the separation mechanism could be speculated by analyzing μeof and μep (Figure 5).
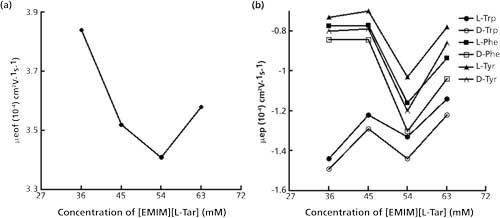
Figure 5: Effects of the [EMIM][L-Tar] concentration on μeof and μep. Conditions were the same as in Figure 1. Acetone was applied to mark EOF.
As displayed in Figure 5b, the differences of μep between each pair of enantiomers first increased then decreased, which agreed with the changes of Rs (Figure 1a). The value of μep was negative. This was related to the isoelectric point of the tested amino acid. For example, the isoelectric points of Trp, Phe, and Tyr are 5.89, 5.48, and 5.66, respectively. Hence, for [EMIM][L-Tar] at pH 8.5, the enantiomers were anions and migrated more slowly than acetone.
As shown in Figure 5a, when the concentration of [EMIM][L-Tar] increased from 36 to 63 mM, μeof first decreased from 3.84x10-4 to 3.41x10-4 cm2V-1s-1, then rose to 3.58x10-4 cm2V-1s-1. The mechanism of enantioseparations was deduced in Figure 6. Generally, the capillary wall carries negative charges because of the silanol groups, and build-up of cations takes place to counterbalance those negative charges. Upon the application of a positive voltage, these cations ,together with their surrounding hydrating water, will migrate towards the cathode, which causes the whole solution to be dragged forward and generates EOF (33). Looking at Trp enantiomers and [EMIM][L-Tar], for example in Figure 6a, when low concentration of [EMIM][L-Tar] was introduced into the capillary, some free [EMIM] cations adsorbed onto the capillary wall and attracted anions, which would suppress EOF. Meanwhile, other free [EMIM] cations combined with L-Tar anions to form [EMIM][L-Tar], which formed ternary complexes by two steps. One step was to form binary complexes, the other was to form ternary complexes (Figure 6). Moreover, other L-Tar anions combined with H+ to form L-Tar, which further formed ternary complexes by two steps. Hence, with an increase in concentration, more free [EMIM] cations adsorbed onto the capillary wall, which led to prolonged migration time and decreased EOF (Figures 1b and 5a). However, when the concentration reached a certain value, the increased concentration caused increased EOF and a short migration time. This could be due to the increasing L-Tar anions. When the concentration was much higher, there were excessive free L-Tar anions capable of combining with [EMIM] cations adsorbing on the capillary wall. Hence, the effects of [EMIM] cations on EOF would be eliminated to some extent, resulting in the increase of μeof (Figure 5a).
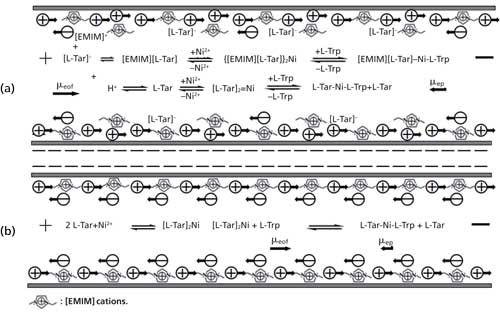
Figure 6: Mechanism of enantioseparations using (a) [EMIM][L-Tar] and (b) L-Tar with EMIM-Ace.
As shown in Figure 6b, when using L-Tar as the chiral ligand, EMIM-Ace was only used to suppress EOF. The [EMIM] cations here did not combine with L-Tar anions, hence low concentration of EMIM-Ace would obviously decrease EOF and prolong the migration time (Figure 4b). This indicates that [EMIM][L-Tar] reacted as a whole in CLE-CE. If only the L-Tar part of [EMIM][L-Tar] was involved in the reaction, [EMIM] cations at such high concentration would tremendously decrease EOF and prolong the migration time. Our research found the opposite. When the concentration of [EMIM][L-Tar] was 63.0 mM, poor enantioseparation was obtained and the migration time of Trp enantiomers was between 8–10 min, which was shorter than illustrated in Figures 3c and 4a–b. Therefore, the main reactions in Figure 6a were reactions of [EMIM][L-Tar] to form [EMIM][L-Tar]-Ni-L-Trp, while secondary reactions were reactions of L-Tar to form L-Tar-Ni-L-Trp.
In conclusion, the effect of chiral ionic liquids on enantioseparations in CLE-CE is not a simple superposition of the effect of cations and anions, but a complex one. Compared to simple chiral ligands, [EMIM][L-Tar] has better enantioseparation ability, which could be due to its larger structure and thus larger differences between ternary complexes.
Conclusion
[EMIM][L-Tar] was introduced for the enantioseparations of Trp, Phe, and Tyr enantiomers by CLE-CE. After optimizing the separation conditions, good enantioseparations were achieved. By comparison with L-Tar and [EMIM][L-Pro], [EMIM][L-Tar] was proven to be a good chiral ligand for enantioseparations of amino acids. The mechanism study indicated that the chiral ionic liquid reacted as a whole in CLE-CE, which brought bigger differences between ternary complexes and thus larger resolutions. Finally, the effect of the chiral ionic liquid on enantioseparations is not a simple superposition but a complex one.
Acknowledgments
The present research was financially supported by National Nature Sciences Funding of China (Numbers 81202913 and 21405075), Natural Sciences Funding of Fujian Province, China (Numbers 2013Y0057 and 2013J01052), Young Teachers’ Teaching and Scientific Research Project of Fujian Province, China (Numbers JA14248 and JA14257).
References
- L. Huang and G. Chen, Anal. Meth.3, 488–508 (2011).
- L. Huang, J.-M. Lin, L. Yu, L. Xu, and G. Chen, Electrophoresis30, 1–7 (2009).
- S. UzaÅçı and F.B. Erim, J. Chromatogr. A1338, 184–187 (2014).
- J. Zhou, F. Ai, B. Zhou, J. Tang, S.-C. Ng, and W. Tang, Anal. Chim. Acta800, 95–102 (2013).
- V. Maier, K. Kalíková, A. PÅibylka, J. Vozka, J. Smuts, M. Švidrnoch, J. ŠevÄík, D.W. Armstrong, and E. TesaÅová, J. Chromatogr. A1338, 197–200 (2014).
- A.P. Kumar and J.H. Park, J. Chromatogr. A1218, 1314–1317 (2011).
- J. Chen, Y. Du, F. Zhu, and B. Chen, J. Chromatogr. A1217, 7158–7163 (2010).
- J. Haginaka, J. Chromatogr. A875, 235–254 (2000).
- S.A.A. Rizvi and S.A.Shamsi, Anal. Chem.78, 7061–7069 (2006).
- H. Zhang, L. Qi, J. Qiao, and L. Mao, Anal. Chim. Acta691, 103–109 (2011).
- M.G. Schmid and G. Gübitz, Anal. Bioanal. Chem.400, 2305–2316 (2011).
- M.G. Schmid, O. Lecnik, U. Sitte, and G. Gübitz, J. Chromatogr. A875, 307–314 (2000).
- H. Hödl, M.G. Schmid, and G. Gübitz, J. Chromatogr. A1204, 210–218 (2008).
- L. Qi, G. Yang, H. Zhang, and J. Qiao, Talanta 81, 1554–1559 (2010).
- D. Rizkov, S. Mizrahi, S. Cohen, and O. Lev, Electrophoresis31, 3921–3927 (2010).
- C. Kuo, K. Liao, Y. Liu, and W. Yang, Molecules16, 1682–1694 (2011).
- J.L. Anderson, D.W. Armstrong and G.-T. Wei, Anal. Chem.78, 2892–2902 (2006).
- M.J. Earle, J.M.S.S. Esperanca, M.A. Gilea, J.N.C. Lopes, L.P.N. Rebelo, J.W. Magee, K.R. Seddon, and J.A. Widegren, Nature439, 831–834 (2006).
- M. López-Pastor, A. Domínguez-Vidal, M.J. Ayora-Cañada, B.W. Simonet, B. Lendl, and M. Valcárcel, Anal. Chem.80, 2672–2679 (2008).
- T.D. Ho, H. Yu, W.T.S. Cole, and J.L. Anderson, Anal. Chem.84, 9520–9528 (2012).
- C. Ragonese, D. Sciarrone, P.Q. Tranchida, P. Dugo, G. Dugo, and L. Mondello, Anal. Chem.83, 7947–7954 (2011).
- D.E. Siyutkin, A.S. Kucfierenko, M.I. Strachkova, and S.G. Zlotin, Tetrahedron Lett.49, 1212–1216 (2008).
- L. Zhang, S. Luo, X. Mi, S. Liu, Y. Qiao, and J. Cheng, Org. Biomol. Chem.6, 567–576 (2008).
- J. Ding, T. Walton, and D.W. Armstrong, Anal. Chem. 76, 6819–6822 (2004).
- W. Bi, M. Tian, and K. Row, Analyst136, 379–387 (2011).
- F. Tang, Q. Zhang, D. Ren, Z. Nie, Q. Li, and S. Yao, J. Chromatogr. A1217, 4669–4674 (2010).
- Q. Liu, K. Wu, F. Tang, L. Yao, F. Yang, Z. Nie, and S. Yao, Chem.-Eur. J.15, 9889–9896 (2009).
- X. Mu, L. Qi, Y. Shen, H. Zhang, J. Qiao, and H. Ma, Analyst 137, 4235–4240 (2012).
- X. Mu, L. Qi, H. Zhang, Y. Shen, J. Qiao, and H. Ma, Talanta97, 349–354 (2012).
- H. Zhang, L. Qi, Y. Shen, J. Qiao, and L. Mao, Electrophoresis34, 846–853 (2013).
- Z. Zheng, Y. Wei, and J.-M. Lin, Electrophoresis25, 1007–1012 (2004).
- D. Wang, Z. Li, L. Wang, C. Qu, and H. Zhang, Chromatographia70, 825–830 (2009).
- S.F.Y. Li and Y.S. Wu, Capillary Electrophoresis (Academic Press, Boston, Massachusetts, 2000), pp. 210.
Lu Huang, Yi-Ting Chen, and Yan-Xia Li are with the Department of Chemistry and Chemical Engineering at Minjiang University in Fuzhou, China. Li-Shuang Yu is with the College of Pharmacy at Fujian University of Traditional Chinese Medicine in Fuzhou, China. Direct correspondence to: luhuang@mju.edu.cn

New Study Investigates Optimizing Extra-Column Band Broadening in Micro-flow Capillary LC
March 12th 2025Shimadzu Corporation and Vrije Universiteit Brussel researchers recently investigated how extra-column band broadening (ECBB) can be optimized in micro-flow capillary liquid chromatography.
A Review of the Latest Separation Science Research in PFAS Analysis
October 17th 2024This review aims to provide a summary of the most current analytical techniques and their applications in per- and polyfluoroalkyl substances (PFAS) research, contributing to the ongoing efforts to monitor and mitigate PFAS contamination.
Characterization of Product Related Variants in Therapeutic mAbs
October 15th 2024Navin Rauniyar and Xuemei Han of Tanvex Biopharma USA recently discussed how identifying product-related variants through characterization enables the recognition of impurities that compromise the quality and safety of drugs.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)