Considerations on Switching from Helium to Hydrogen
LCGC North America
The authors explain the operational considerations for switching from helium to hydrogen in the separations lab, as well as discussing options.
Gas chromatography (GC) uses a wide range of gases including helium, hydrogen, and nitrogen as the carrier gas. In the U.S., the most common carrier gas is helium, as it provides good separations, is inert, is readily available, and it is safe to use. In many other countries, helium is less available or is very expensive, and hydrogen is commonly employed. In recent years, the availability of helium has decreased and its cost has increased significantly, so many chromatographers have considered switching to the use of hydrogen (1). In this article, we compare the use of helium and hydrogen in GC and discuss the advantages of hydrogen. In addition, we will discuss how a chromatographer can obtain hydrogen in a convenient, safe, and economical manner to meet the needs of the laboratory.
The Availability of Helium
Helium is a minor component of natural gas formed by the natural breakdown of uranium. Fractional distillation of natural gas allows for the purification of helium from the natural gas. The largest helium concentrations found in natural gas are in Texas, Oklahoma, and Kansas. Sites outside the U.S. with high concentrations of helium are in Algeria and Qatar.
Helium has many uses and applications. These include (by order of size) cryogenic cooling (28%), tank purging (26%), welding cover gas (20%), as a controlled atmosphere (13%), leak detection (4%), and for breathing mixtures (2%) (2). The use of the gas as a carrier gas for GC falls under a miscellaneous category and is a relatively small fraction of the available supply. Many larger users of helium are seeing considerable growth in their use of the gas and will need even larger quantities in the future.
Helium is a critical and national resource for the U.S. During World War II, huge caverns were sealed and used to store the helium, creating the USA Helium National Reserves. In 1995, the government decided to allow the sale of 600 million ft3 of helium between January 1, 2005 and January 1, 2015 (The Helium Privatization Act of 1996- Public Law 104-273) (3). Sales are ongoing and continue in the U.S. and to foreign countries, leading to reduced supplies and much higher prices for helium. In addition, breakdowns in manufacturing facilities have also reduced available supplies (4). The end result of the increase in demand and the decrease in supply has required the withdrawal of the gas from the national reserves and there are significant concerns about the ability to obtain helium on a reliable basis. Many users worry more about the ability to obtain helium when they need it than the increased price.
Why Use Hydrogen for Gas Chromatography
Hydrogen and helium are both very efficient gases to use for GC and chromatographers can switch from one to the other with little to no difficulty. While the nature of the separation is the same with the two gases, the differences in the properties of the two gases will lead to differences in the efficiency of the separation. A plot of the efficiency of a column is a van Deemter (4) (packed column) or Golay (5) (capillary column) plot that depicts the efficiency of a column versus different operating velocities of various carrier gases. The van Deemter equation (equation 1) is used to calculate the height equivalent to a theoretical plate (HETP). It describes the column efficiency or a measure of its ability to separate peaks. The desired HETP value would be the smallest value obtainable.
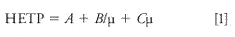
where
µ = linear velocity of carrier gas (mobile phase)
A = A constant that accounts for the effects of "eddy" diffusion in the column. (The A term is not used with capillary columns because there is only one flow path and no packing material in a capillary column)
B = A constant that accounts for the effect of molecular diffusion of the vapor in the direction of the column axis
C = A constant proportional to the resistance of the column to mass transfer of solute through it.
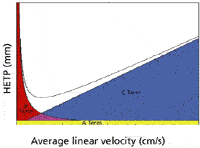
Figure 1: Plot of HETP versus linear velocity (5).
Figure 1 shows a plot of HETP versus linear velocity and depicts the areas of the plot that represent the A, B, and C terms

where
L = Length of the column in centimeters
tM = Retention time in seconds of a nonretained compound (typically methane).
The B term in the van Deemter expression defines the slope of the curve after the minimum (or bottom) or lowest HETP value. It is this slope and the minimum that displays the difference between the use of hydrogen and helium.
From an operational perspective, the HETP values are determined experimentally at various carrier gas velocities. A plot of HETP versus the linear velocity is generated using equation 3 and equation 4.

where
L = Length of the column in centimeters
Neff = Theoretical plate number
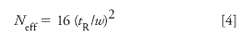
and where
tR = Retention time of the peak being measured
w = Width of the peak.
Measure the peak width for packed columns at the base line and for capillary columns at the half height of the peak.
Determination of HETP
The chromatographer would desire conditions that provide the smallest value for HETP, as this would provide the accumulation of the most plates or optimize the separation power within a column. When looking at the typical plot of HETP versus the linear gas rate (LGR), there is not much difference between the minimum value for the HETP for helium and hydrogen, but there is a large difference for the average linear velocity or linear gas rate values obtained for the HETP minimum for each gas. When using helium with typical capillary columns, the usable range of LGR is 20–30 cm/s, while for hydrogen, we see a much wider range of LGR of 25–65 cm/s. The slope of the HETP versus LGR curve for hydrogen (C term) is smaller than that for helium and shows only a 25% reduction in the optimum HETP value over the 25–65 cm/s range.
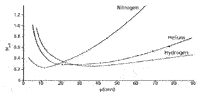
Figure 2: Typical plot for various carrier gases with capillary columns (6).
Switching from Helium to Hydrogen:
When you switch from helium to hydrogen, there are two options:
- Choose to duplicate your analysis using hydrogen with little to no loss of efficiency.
- Choose to take advantage of the higher LGR available with hydrogen to speed up your analysis as the higher linear velocity used for hydrogen leads to shorter retention times and a shorter analysis.
Comparing the Use of Hydrogen and Helium at the Same LGR: The chromatograms in Figure 3 compare the separation of a complex sample of bacterial acid methyl esters on an Equity-1 column with a linear velocity of 25 cm/s for both helium and hydrogen. Notice that at the same LGRs that even with this complex sample the retention times for these components are about the same.
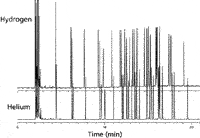
Figure 3: Separation of bacterial acid methyl esters (6).
Comparing Chromatograms at Optimum LGR: The chromatograms shown in Figure 3 shows the separation of bacterial acid methyl esters for helium and hydrogen with the helium being at the optimum LGR for helium and the run for hydrogen also being at 25 cm/s but not at its optimum (hydrogen is the top chromatogram and helium is the bottom chromatogram.) The run shown for hydrogen shows a total run time of 19.5 min. What would this run look like at the optimum of 50 cm/s with hydrogen?
The chromatogram in Figure 4 is the same column run at 50 cm/s using hydrogen at the same program rate as used in Figure 3. The total run time is at 16 min. Though there is an improvement in the reduction of the run time, you do not see a great reduction. This is because this separation is very temperature sensitive and needs a higher temperature program rate to speed it up. To obtain a faster separation you would increase your temperature program rate by close to twice that used for the run in Figure 3. (See later in this article for temperature program concerns.)
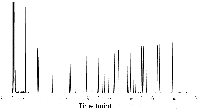
Figure 4: Separation of bacterial acid methyl esters using hydrogen (50 cm/s) (6).
Major Benefits of Hydrogen: The use of hydrogen provides the chromatographer with a number of benefits:
- Increased speed: Increasing the linear flow rate allows for shorter run times, thereby increasing the throughput of a laboratory.
- Achieve lower temperature separations: At the faster elution times, it might not be necessary to increase the column temperature run rate. You might be able to lower the maximum temperature needed for the analysis or remain at those temperatures for shorter periods.
- Longer column life: Lower temperatures lead to less column bleed and can provide longer column life. In addition, hydrogen is a reducing gas and can remove potential acidic sites inside of the column. The removal of these sites leads to less sample absorption and less phase breakdown (column bleed). The result is a longer usable life for the column.
- Environmental concerns: Hydrogen is available readily via the electrolysis of water and is not a critical national resource. In contrast, helium is a by-product of natural gas or petroleum production and there are environmental concerns with the production and purification of the gas. Hydrogen is a green gas in that its production does not contribute to environmental pollution.
- Cost savings: The cost of hydrogen is significantly lower than that of helium. (Comparisons of cost are best made in-house due to differing cost structures from site to site.)
- Availability: Because the generation of hydrogen is from water, the chromatographer need not be concerned about availability issues.
Switching to Hydrogen: Chromatographers can switch to hydrogen readily if flame ionization detection (FID) or other flame-based detection methods such as phosphorus, nitrogen, electron-capture, or Hall detection are employed for the separation. If the separation requires a helium detector, it will be necessary to maintain the use of helium. The use of mass spectrometry (MS) might allow or not allow the use of hydrogen and it is best to check with the manufacturer of your unit about its capability. Thermal conductivity detection (TCD) typically uses helium for the best thermal differences between the carrier gas and other compounds to be detected. Some analyses do use hydrogen as a carrier such as with oxygen, helium, and other very light gases. Therefore, the use of hydrogen for TCD is application dependent.
Delivering Hydrogen to the Chromatograph: When switching the carrier gas from helium to hydrogen, it might be necessary to change the external tubing connections to the GC system. If the GC system employs a flame-ionization detector, a hydrogen line is already in place. First, vent the line and then cut it and install a tee. Connect the other side of the tee to the supply carrier gas.
If you are using copper tubing for delivery of the carrier gas (as is common in many facilities), replace it with stainless steel tubing as copper tubing will oxidize and harden with time. Hardened copper tubing is quite brittle and may break if it is bumped while stainless steel tubing is much more robust. To avoid contamination to the GC system be sure to use clean, preferably GC-quality tubing.
The purity of the fuel gas also should be a consideration. If fuel-grade hydrogen is used, it will be necessary to include a purifier in the system to reduce moisture and oxygen levels so that the gas is 99.9999% pure. The other choice is to switch to carrier-gas grade as the carrier gas. Several inline purifiers are available that will reduce the impurities to the desired levels and include indicators to notify the user when the purifier is expended and needs to be changed.
If the GC system automatically adjusts for LGR: Many modern GC systems include electronic pneumatics and it simply necessary to indicate the gas used so that automatic adjustments based on the density differences of the gases are considered. Indicate that hydrogen is the carrier gas in the control program of the GC system and the unit will make the necessary density adjustments in controlling the carrier gas. Typically, the system will control the LGR in the column, the split ratio, and the amount of flow of fuel gas to the FID system.
If you are using a short column and a flow controller: Some units use flow controllers in combination with pressure controllers to control the LGR. If you are using a short column or wide bore column, the LGR for hydrogen might lead to a column head pressure that is below 10 psig. In this case, it might be necessary to change the flow controller to allow flow control below 10 psig.
If the flow of the system is controlled by pressure: The operation of simple GC systems and some older GC systems is related to the pressure of the carrier gas (that is, do not automatically adjust for LGR); in this case, it will be necessary to make adjustments to the head pressure. To achieve the same LGR for hydrogen as you used for helium, it will be necessary to set the head pressure to approximately 45% of the pressure used for helium.
Changes needed for various injection techniques: Adjusting the split ratio: If you desire to shorten your analysis time by increasing your LGR, then you will need to reestablish your vent flow to maintain your desired split ratio. This adjustment to the split ratio is necessary to allow for only the correct amount of sample to enter the column; the rest of the sample goes to a vent. The ratio of sample entering the column versus that going to the vent is the split ratio. The typical injection size of 1 µL of sample saturates most columns and leads to broad peaks. splitting the sample reduces the amount of sample entering the column to an acceptable level. When switching the carrier gas to hydrogen, it may be necessary to adjust the split ratio (which is the ratio of sample and carrier gas that enters the column versus how much is vented) and increase the LGR to obtain the best possible analysis and the shortest possible run time.
Split ratios: To calculate the split ratio you most know the flow of the column and the split vent flow. With this technique, it is desired to reduce the amount of sample down to a level where the on-column concentration of the individual components due not saturate and cause wide and often tailing peaks.
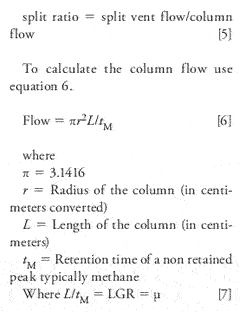
Simplified:
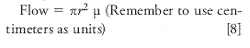
With a column that has an internal diameter of 0.25 mm, the split ratio is typically 100/1 (that is, only 1 part in 100 of the sample enters the column and the rest of the sample and carrier gas flow is vented).
Always measure the column flow and vented carrier gas before changing the gas to make sure you know the split ratio you were using. After changing the gas, it will be necessary to measure and adjust your split vent flow to allow for the same split ratio you previously used.
Note: The split flow does not always need to be on. With splitless injection techniques, the flow is off for a short period after injection. Use this same technique with a split injection technique. After about 2 min, the entire sample that is going into the column is in the column, and the split flow can be turned off at this time. Turn it back on as you prepare for the next injection. This can provide huge savings in gas usage.
Note: If you are doing splitless injections you will still need to know the amount of gas exiting the vent port when the port is open (typically 20–60 s after an injection). Make adjustments to duplicate the split flow after the switch to hydrogen.
Splitless injection techniques: Hydrogen is preferred over helium for splitless injection techniques as it carries the solute from the inlet into the column faster than helium. This results in sharper peaks (higher efficiencies) and reduces band broadening with the resulting wide and shorter peaks, which allows for lower detection limits.
Direct injection: There is no concern on the conversion when using direct injection.
Temperature Programming Concerns
Many analyses use temperature programming to allow the later eluted compounds to be eluted at a reasonable temperature to obtain sharp symmetric peaks. The changeover from helium to hydrogen has significant implications with temperature programming runs. There is a significant difference in the viscosity of helium and hydrogen and this difference is temperature dependent as indicated in Figure 5. Both gases increase in viscosity as temperature increases, but hydrogen has a much lower viscosity than helium throughout the temperatures shown. The lower viscosity of hydrogen means that lower pressures are required for hydrogen. Because the viscosity of hydrogen is approximately 45% lower than helium, the pressure needed to run the analysis is about 45% lower when hydrogen is used. When running capillary columns with a system using LGR, you can use the same LGR but the resulting head pressure for the hydrogen is 45% lower.
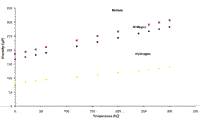
Figure 5: Plot of gas viscosity versus pressure (6).
If you choose to use higher flow rates for hydrogen, it might be necessary to make corrections to the temperature program when desiring a rapid analysis time. Some compounds might be eluted faster but provide broader peaks than desired if the temperature program rates are not increased to match the higher flows. If, for example, when doubling the LGR and using a 5 °C/min temperature program rate, it might be desirable to increase the program to 10 °C/min. If the peaks are coming out rapidly and are sharp, you can take advantage of the lower column temperatures and stop the temperature programming just before or after the last peak is eluted. This might mean a lower final run temperature and will often extend column life.
Elution Order of Peaks: With most analyses, the change of linear gas rate and temperature might not change the order of elution of compounds. If you choose to use the same LGR and temperature as used with helium, typically, you should not see any problems. However, with polar columns, such as Carbowax or the highly polar Cyano phase columns it may be necessary to check the elution order as some column phases, at differing temperature, exhibit different polarities or orders of peak elution.
Detector Optimization Concerns: Flame detectors need an optimum flow of hydrogen to optimize the flame sensitivity and other detectors can have similar requirements on how much hydrogen can be used. With the flame ionization detector, the flow optimum is 30–40 cm3 /min of hydrogen. When you switch to hydrogen as a carrier gas, you will have to take the flow from the column into consideration so that you do not exceed the optimum flow range of hydrogen for the detector. With packed columns or large-bore capillary columns, this might mean reducing the fuel gas flow so that the combination of carrier and fuel gas is at that rate previously used for the separation. For typical detector fuel flow ranges see Table I.

Table I: Typical detector fuel gas flow rates*
Make-Up Gas for Detectors: If you do use hydrogen as the make-up gas, it will be necessary to consider this flow with the carrier gas and fuel gas to optimize the detector sensitivity. Take care not to saturate the detector with too much hydrogen, as this will affect both base line noise and sensitivity.
Hydrogen is not the best choice for make-up gas; the best gas for a make-up gas with flame detectors is 99.9999% pure nitrogen. Its use leads to lower baseline noise and better flame performance.
Safety Concerns When Using Hydrogen
General Considerations: Hydrogen gas is used commonly in the laboratory for a variety of purposes and is the carrier gas of choice for gas chromatography in countries other than the U.S. It is the fuel used in the most commonly used detectors (flame ionization, nitrogen, and phosphorus detectors) and therefore, already in the laboratory and in use with most gas chromatographs.
If the analyst changes from helium to hydrogen, the safety issues should be well understood to ensure safe operation. The flammability of the gas ranges from 4 to 74% in air, with an explosion limit of 18.3–59%, so there are real hazards if a build-up of hydrogen were to occur. Similarly, if a large buildup of hydrogen or helium were to occur in the laboratory, the oxygen concentration for breathing might be compromised. As we describe in the following, the use of a hydrogen generator obviates many of the safety concerns, as only a small quantity of gas is present at a given time, in contrast a significant amount of hydrogen is present when a tank is used.
Although hydrogen can form an explosive mixture with air, it diffuses rapidly. It dilutes quickly by combining with air into a nonflammable concentration. Hydrogen rises two times faster than helium at a speed of almost 45 mph (20 m/s). In a laboratory with good air turnover, it would be very difficult to achieve flammable limits. In addition, most modern GC systems incorporate a turn-off system when flows or pressures suddenly increase or decrease (as in the case of a column breakage in the GC oven) and help to reduce the possibility of a problem.
In the case of a column or line break when using a gas generator, the available volume of gas is low, and also the generation of gas will be terminated. Generators only store about 60 cm3 of gas.
Proper Venting: It is best to consider special venting systems to remove the chance of any build up of hydrogen. When using hydrogen as a carrier gas you need to consider all of the flows exiting the unit. In many detectors, the carrier gas burns in the flame and is not a problem, but you should consider the venting of the gas from detectors that do not burn the hydrogen.
A source of venting is the septum vent port. The typical septum purge is 1–5 cm3/min. It is easy to vent this flow with the split vent exhaust. The split exhaust port will have the highest vented flow. split vent ports can have flows of 50–500 cm3/min. This can mean a lot of vented hydrogen and will need special venting.
Many laboratories already have special fume hood vents over the split vent to allow for the venting of hazardous sample components. If you do not have such vents in place, you should consider them for both the sample and the venting of hydrogen.
Safety Concerns: Gas Generators Versus Cylinders: The concern with flammable gases is with the build up of these gases to flammable or explosive limits. Consider as one of the primary concerns the total volume of hydrogen in your lines. If a break in the line occurs, an explosive level of the gas in the lab could be present. Gas generators with their safety shutoffs and monitoring safety features only allow for small volumes of gas in their lines and units. If there is a sudden release of pressure or flows, the gas generators will turn off (some modern GC systems also incorporate this feature).
Cylinders often involve long lines leading to GC systems and the hook-ups are often at the end of benches or in other rooms. With a long run of tubing, you will have large volumes of gas in the lines, under pressure, and the possibility of venting of these lines with a line break. This could allow for the entire venting of the volume of one or more cylinders into the lab. With the proper installation of cylinders, it is unlikely that you will see cylinders taking off like rockets and shooting across laboratories. Be sure to review your operating procedures and safety concerns before switching to hydrogen. There are codes and standards for safe building and installation practices. Any new hydrogen component installations should follow strict guidelines and undergo third-party testing for safety and structural integrity. To make sure you have the latest guidelines you can check your standards against the following sources on the Internet.
Internet sources of safety information include www.hydrogenSafety.info; www.fuelcellstandards.com; and www.eere.energy.gov/hydrogenandfuelcells/codes.
Ways of Supplying Hydrogen Gas via an In-house System: To obtain hydrogen gas for GC, use high-pressure cylinders or an in-house generator. The in-house generation of hydrogen is based upon the electrolysis of water, as shown in equation 9.
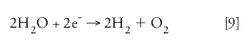
Connecting a power source to two electrodes placed in water performs the electrolysis of water. The cathode, where hydrogen gas collects can be a metal electrode or a polymeric membrane, while the anode, where oxygen is collected, is a metal electrode.
Electrolysis of water using two metal electrodes: The electrolysis of water can be performed readily using a metallic cathode and metallic anode immersed in a strong, water-soluble electrolyte such as 20% sodium hydroxide. The base is an electrolyte, as pure water does not conduct a current very effectively, and the quantity of hydrogen generated is very low. To provide hydrogen of high purity, the cathode consists of a bundle of palladium tubes. The cathodes are palladium tubes because only hydrogen (and its isotopes) is capable of passing through it and ultra-high-purity hydrogen gas is obtained.
As an alternative to the use of a palladium cathode, some systems that generate hydrogen via the electrolysis of water via a metal electrode use a stainless-steel cathode and employ a desiccant as the final drying agent. While the initial cost of such approach is lower, the hydrogen gas collected in this manner is less pure as it contains significantly more oxygen and nitrogen than hydrogen gas generated via a palladium electrode (see Figure 6). In addition, systems that employ a desiccant, require regeneration on a periodic basis.
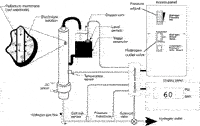
Figure 6: Flow schematic of a hydrogen generator with a palladium membrane.
Presented in Figure 6 is a typical hydrogen generator (Parker Balston Model H2PD-300, Parker Hannifin Corporation, Haverhill, Massachusetts). It generates hydrogen via the electrolysis of water using a metal electrode. This system generates hydrogen with a purity of 99.99999+%, oxygen content of <0.01 ppm, and moisture content of 0.01 ppm at a maximum flow rate of 300 mL/min with a maximum outlet pressure of 60 psig.
Electrolysis of water using a proton exchange membrane: In recent years, ionic polymeric materials such as Nafion (a sulfonated tetrafluoroethylene polymer) or polybenzimidzole (PBI) have been found to conduct protons while being impermeable to gases such as hydrogen and oxygen. Proton exchange membranes (PEMs) made from such polymers are used in fuel cells to generate electricity from oxygen and hydrogen. If a potential is applied to a system containing a PEM in the presence of water and a counterelectrode, the water will be dissociated to form hydrogen ions, which are then converted into hydrogen gas. The primary benefit of using a PEM is that deionized water can be used instead of the 20% sodium hydroxide used when two metallic electrodes are used and sodium hydroxide, which is caustic, is not required. When a PEM is used to generate hydrogen, a palladium membrane can be used to purify the hydrogen further by reducing the oxygen concentration to less than 0.01 ppm and moisture down to <1.0ppm.
Shown in Figure 7 is the general design of a hydrogen generator based upon PEM membrane technology (Parker Hannifin Model H2PEM-510). This system is capable of generating 99.9995% pure hydrogen (noncarrier grade) at a flow rate of 510 mL/min at pressures up to 100 psi.
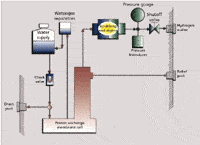
Figure 7: Hydrogen generator with a proton exchange membrane.
Figure 8 shows the chromatograms of hydrogen gas collected via a gas chromatograph equipped with a discharge ionization detector. The red trace is from gas generated via a palladium cathode, while the black trace is from gas collected using a stainless-steel electrode and a desiccant drying tube.
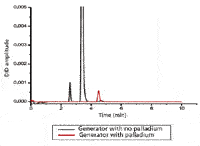
Figure 8: Hydrogen collected via a GC system equipped with a discharge ionization detector.
The large black peaks indicate the presence of a combined concentration of 12 ppm of O2 and N2 in the hydrogen, which is not present in the hydrogen that was dried with the Pd tubes. It is clear that the palladium tube cathode provides a very considerable improvement in purity.
Conclusion
The process of switching from helium to hydrogen involves many issues but with attention to detail, one can switch successfully and duplicate previous analysis with little to no problems. If desired, the analysis can be run at higher LGRs and greatly reduce the analysis time. The switch from helium to hydrogen will allow reduced cost of analysis due to the lower cost of hydrogen and allow for longer column lifetimes. For most chromatographers, hydrogen is a gas already in the lab and is not an increased safety issue. Consider venting hydrogen, along with any sample vapor, to provide the safest, cleanest environment for the analyst to work within. To avoid the problems with cylinders in the laboratory and to obtain the best, most convenient source of hydrogen, consider the use of a hydrogen gas generators.
Reginald Bartram is presently a consultant for gas chromatography and is semiretired. He is a past president of the Chromatography Forum of Delaware Valley and the recipient of the 1999 Chromatography Forum of Delaware Valley Award for Contribution to Theory, Instrumentation, and Application of Chromatography. He has been widely published on the subject of gas handling and gas purification. In his 35 years of service to GC, he has worked at Supelco and Alltech as a scientist and marketing manager.
Peter Froehlich is President of Peak Media, Franklin, Massachusetts. He received the Ph.D. in chemistry from Purdue University, West Lafayette, Indiana. He has over 30 years of marketing and technical support experience in the scientific instrument industry, with an emphasis on a broad range of chromatographic techniques including GC and LC.
References
(1) N. Pacheco, U.S. Bureau of Mines, U.S Geological Survey Minerals Yearbook, 1 –2, (2007).
(2) The Helium Privatization Act of 1996, Public Law 104-273.
(3) M Rose, Photonics 1–12, 2008.
(4) J.J. van Deemter, F.J. Zuiderweg, and A. Klinkenberg, Chem. Eng. Sci. 5, 271 (1956).
(5) E. Glueckauf, M.J.E. Goley, and J. H. Purnell, Ann NY Acad. Sci. 72, 612 (1956).
(6) L. Sidisky, "Carrier Gas Selection: Helium vs. Hydrogen," Pittcon 2008, Orlando, Florida (Slides 12, 21, 46–48, 53, 54).

Study Examines Impact of Zwitterionic Liquid Structures on Volatile Carboxylic Acid Separation in GC
March 28th 2025Iowa State University researchers evaluated imidazolium-based ZILs with sulfonate and triflimide anions to understand the influence of ZILs’ chemical structures on polar analyte separation.












