Chromatographic Comparison of HILIC Stationary Phases Prepared Using Various Hydrophilization Techniques
Selectivity of hydrophilic interaction liquid chromatography (HILIC) stationary phases is crucial for choosing a column for separating target polar compounds, which is why it is important to know the effect of each functional group on the chromatographic properties of the material. Revealing the trends in selectivity changes with variations of adsorbent structure at different stages of preparation can help to regulate the interactions of the stationary phase with analytes and create novel materials with improved performance. This article compares several silica-based HILIC stationary phases with different types of coatings, including polar polymers, antibiotic macromolecules, zwitterionic, and amide groups. Synthesized adsorbents were characterized and compared using the Tanaka test for hydrophilic stationary phases. Their chromatographic performance was evaluated using model mixtures of neutral, zwitterionic, and negatively and positively charged polar analytes. The obtained results provided better understanding of the factors affecting stationary phase selectivity.
Synthesis of stationary phases for hydrophilic interaction liquid chromatography (HILIC) utilizes different techniques where a hydrophilic substrate, mostly silica, is covered with hydrophilic coating containing neutral or charged functional groups of different nature. The structure of those groups, as well as their arrangement in the layer relative to each other (as separate functional sites or uniform polymer layer), defines chromatographic performance of stationary phases in HILIC mode and their ability to retain and separate various classes of polar analytes.
Our research group synthesized and described HILIC stationary phases with various coatings including monomeric amide ligands prepared via the Ugi reaction (1,2), linear polymers (3,4), glycopeptide antibiotic (5), and a zwitterion (6) (Table I). Functional groups were attached to the substrate either directly or using 1,4 butanedioldiglycidyl ether (1,4-BDDGE) as a linker.
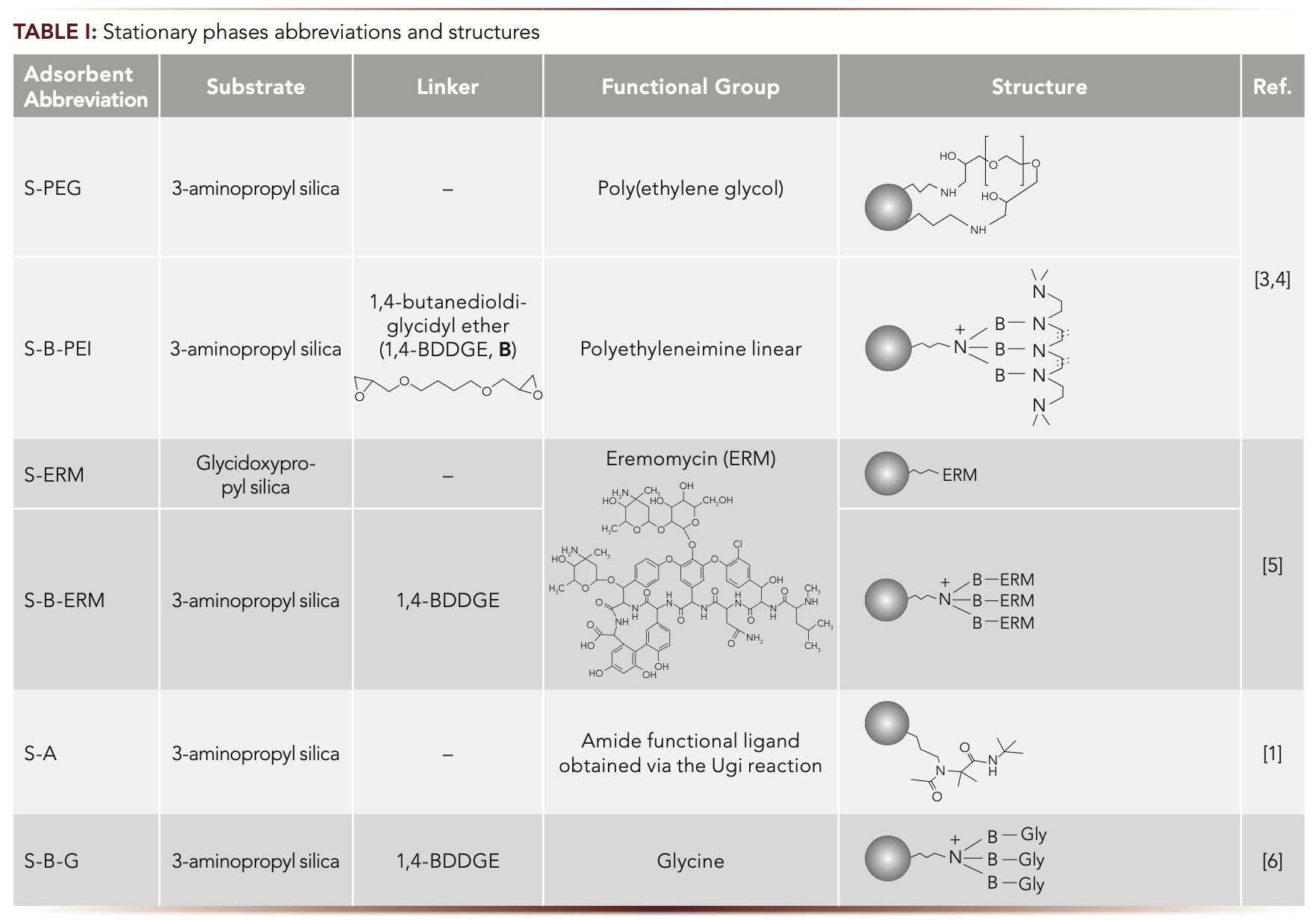
Using polymers is promising for obtaining stationary phases for HILIC. It leads to both hydrophilizing and shielding the substrate and thus decreasing its effect on the retention of the analytes. One of the suitable polymers is poly(ethylene glycol) (PEG), which is easily synthesized with a pre-defined mass polymer with a linear architecture. The presence of oxyethylene groups may lead to dipole–dipole interactions and the potential formation of hydrogen bonds. Polyethyleneimine (PEI) is another polymer which provides both high hydrophilicity and anion-exchange properties, ensuring the simultaneous determination of polar neutral compounds and anions.
Another way of shielding the substrate is attaching macromolecules. Glycopeptide antibiotics such as eremomycin (ERM) contain a lot of amide-, hydroxy-, and amino groups providing hydrophilicity and thus the prospect for obtaining HILIC phases in addition to chiral ones.
A promising approach for the creation of new functional layers for HILIC adsorbents is using multicomponent reactions. Their advantages are the high yield, ease of execution, “one-pot” reaction without isolating intermediate products, and flexibility of structure variations. The multicomponent Ugi reaction involves the interaction of amines, carbonyl compounds, isocyanides, and acids. Simultaneous incorporation of several types of polar functionalities including amides via this reaction can provide hydrophilicity to the phase and variability in its derivatives. The use of aminated silica as an amino component in this reaction proposed in (1,2) makes sure that the substrate is modified exclusively with the necessary functional groups, while possible byproducts of the competing Passerini reaction are formed only in the liquid phase and can be washed out.
Another promising set of HILIC phases are zwitterionic ones. They tend to be the most universal species allowing separation of analytes from different classes. Such a phase was obtained by covalent attachment of glycine to 3-aminopropyl silica via the linker 1,4-BDDGE (6). The structures of the discussed phases are given in Table I.
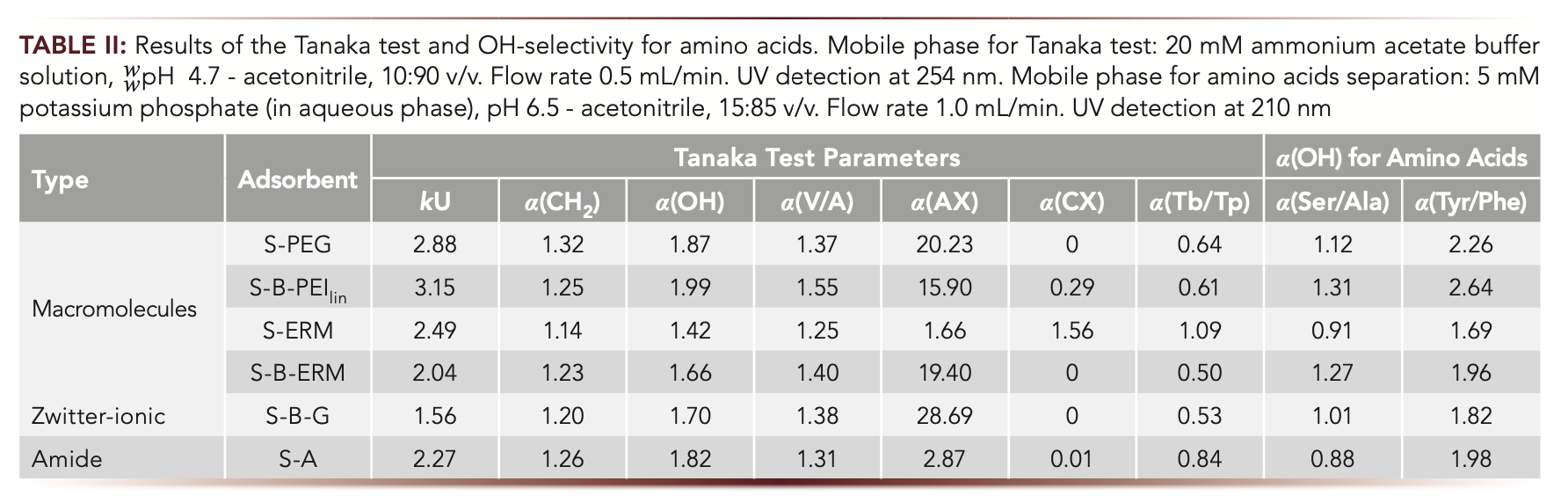
To characterize and compare the synthesized adsorbents, the Tanaka test for hydrophilic stationary phases (7) was used. The set of substances is recommended in the test to quantify the different types of selectivity parameters. Hydrophilicity of the adsorbent surface k(U) is determined as the uridine retention factor. The degree of hydrophobic interaction α(CH2) is represented by selectivity toward uridine and 5-methyluridine k(U)/k(5MU). Hydrophilic partitioning property as selectivity to the hydroxy group α(OH) is defined as k(U)/k(2dU) using 2’-deoxyuridine (2dU). Hydrophobic ions sodium p-toluenesulfonate (SPTS) and N,N,N-trimethylphenylammonium chloride (TMPAC) reflect anion- and cation-exchange properties α(AX) and α(CX), respectively. The phases are also characterized by their ability to separate configurational isomers vidarabine and adenosine α(V/A) and α(Tb/Tp) for theobromine and theophylline, which determine surface pH. Conditions are given in Table II.
In this work, model mixtures of sugars, amino acids, and water-soluble vitamins were also used as hydrophilic compounds representing neutral, zwitterionic, and positively or negatively charged polar analytes. Comparison of the chromatographic performance of the prepared stationary phases and discussion of the modification pathways leading to better selectivity and efficiency toward each class of analytes is presented.
According to the data given in Table II, hydrophobic α(CH2) and configurational isomers selectivity α(V/A) does not differ significantly for the obtained phases. Neutral character for S-ERM is determined since α(Tb/Tp) = 1.09, while basic nature of the other adsorbents is supported with α(Tb/Tp) < 1. Other parameters are discussed further in detail.
Stationary Phase Hydrophilicity
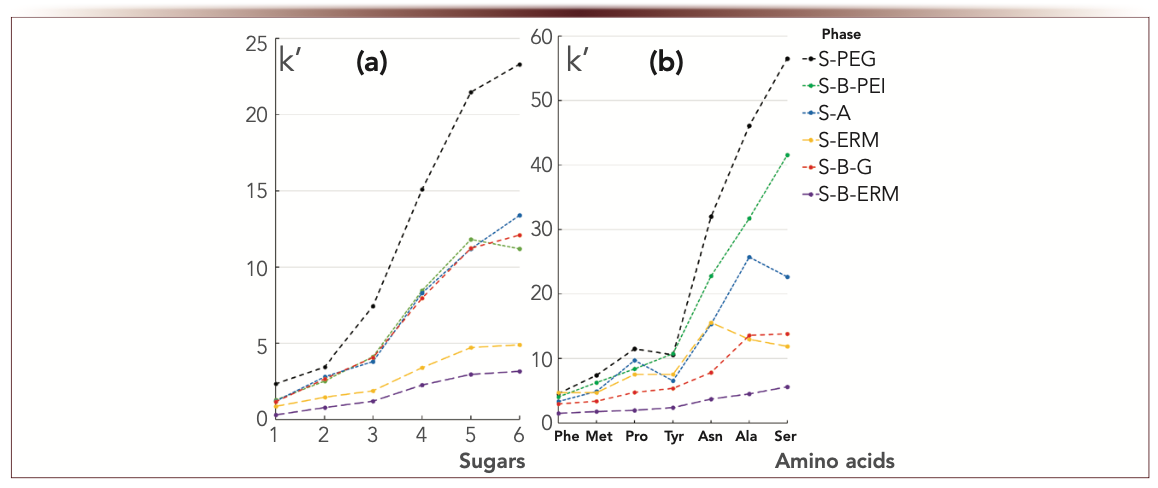
To reveal which of our modification ways allowed to obtain more hydrophilic adsorbents, neutral hydrophilic compounds of different structures were taken. Uridine retention factor was used from Tanaka HILIC test to reflect this characteristic (conditions are given in Table II and [7]). Hydrophilic retention was also evaluated using the set of neutral polar analytes—sugars and zwitterionic amino acids. An appropriate mobile phase composition was used for each model mixture. Acetonitrile and water were taken for sugars. Acetonitrile and phosphate buffer with ww pH 6.5 was used for the amino acids, which is close to their isoelectric points (other details are given in Figure 1).
FIGURE 1: (a) Retention factors for sugars and (b) amino acids. Mobile phase for sugars: deionized water:acetonitrile, 15:85 v/v. Flow rate 1 mL/min. Detection: RID. Columns: 100 × 3-mm i.d. 1 = ribose, 2 = fructose, 3 = glucose, 4 = sucrose, 5 = maltose, 6 = lactose. Mobile phase for amino acids: 5 mM phosphate buffer, wpH 6.5 / acetonitrile, 15:85 v/v. Flow rate 1 mL/min. UV detection at 210 nm. Compounds are indicated for abscissa labels, ordinate label is k’.
According to Table II, hydrophilicity k(U) of the obtained adsorbents measured using the Tanaka test decreases in the following order:
S-B-PEI > S-PEG > S-ERM > S-A > S-B-ERM > S-B-G
Despite the above k(U) values for the phases, the highest retention factors for sugars were registered for S-PEG (Figure 1a). Zwitterionic S-B-G phase with the lowest hydrophilicity k(U) demonstrated strong retention of sugars similar to S-B-PEI with maximum k(U). The lowest sugar k’ values were obtained for the phases modified with eremomycin (S-ERM and S-B-ERM) characterized by moderate k(U) values. These results showed that there is no correlation between hydrophilicity described via k(U) in the Tanaka test and sugars retention. In fact, retention mechanism for carbohydrates is not only partitioning but also involves adsorption and formation of hydrogen bonds with OH- groups. The latter could have greater impact on retention for the phase where PEG containing H-acceptors is attached directly to aminopropyl silica as compared to PEI containing both H-donors and H-acceptors attached via the additional 1,4-BDDGE linker. Also, Schiff base formation is limited for sugars with linear PEI layer containing substituted amino groups. Therefore, it’s not easy to predict carbohydrates’ retention relying only upon the hydrophilicity parameter k(U).
Amino acid retention showed a slightly better correlation with stationary phase hydrophilicity k(U). The lowest amino acid k’ values were registered for the phases S-B-G and S-B-ERM with low k(U) (Figure 1b). S-A showed medium retention values according to its k(U). The highest amino acid k’ values were observed for the most hydrophilic polymer-coated phases, but again S-PEG > S-B-PEI was opposite to their k(U) values. The deviation from the trend could be caused by the effect of functional groups structure and several types of interactions defining the retention of polar analytes.
Therefore, Tanaka HILIC test parameter determining hydrophilic retention k(U) cannot be exclusively applied for reflecting and prediction the retention of polar analytes from different classes between the columns of diverse functionalities and in the cases when different eluting conditions are used. Additional model analytes representing broader structure variations are useful to complement HILIC columns characterization and comparison.
Evaluation of Electrostatic Interactions
Electrostatic interactions have a significant impact on HILIC retention for polar charged analytes. This contribution is evaluated by the slope value for the dependences of their retention factors on the counter-ion concentration in mobile phase. It was determined in (1) for ascorbic and nicotinic acids, where their retention factors decreased with increasing concentration of the eluting ion in the mobile phase. For the phases with different anion- exchange selectivity α(AX), the slope, which reflected electrostatic impact, was identical for the same analyte.
Retention of acidic analytes depend on anion-exchange properties in HILIC mode. To determine anion-exchange selectivity α(AX) in the Tanaka test, a hydrophobic organic anion sodium p-toluenesulfonate (SPTS) was used. Its retention is divided by k(U) to account for the influence of hydrophilic interactions. The mobile phase for this test contains ammonium-acetate buffer with wpH 4.7. It is useful to reveal whether it is possible to predict the retention of charged analytes relying upon α(AX) or α(CX) values, when the elution conditions necessary to ionize target analytes differ from those used in the test. Acidic vitamins were chosen as the test compounds. An ammonium-acetate buffer with wpH 5.8 was used for their separation as it provided the best selectivity within the model mixture of water-soluble vitamins. Also, at this border of the acetate buffer, capacity carboxyl groups of ascorbic and nicotinic acids should be dissociated.
As demonstrated in Table II, all the phases provide anion-exchange selectivity determined as in (8). High α(AX) values were obtained for the phases containing the linker 1,4-BDDGE. This effect is caused by quarternization of nitrogen atoms on the substrate surface when three molecules of 1,4-BDDGE could be attached per each amino group (Table I). α(AX) for S-PEG is also high indicating that terminal epoxy-groups of the initial reagent could also quarternize substrate’s nitrogen atoms. Increased α(AX) values resulted, as expected, in higher retention of the acids. Figure 2 demonstrates the dependence of retention factors for nicotinic and ascorbic acids on α(AX). The coefficient of determination R2 for nicotinic acid was about 0.97 which could be used for approximate prediction of its retention. Lower R2 observed for ascorbic acid (0.86) is consistent with involving different mechanisms in its retention shown in (1).
FIGURE 2: Retention response for negatively (nicotinic and ascorbic acids) and positively (thiamine) charged vitamins on the anion-exchange selectivity of the columns. Mobile phase: 100 mM ammonium acetate buffer, wpH 5.8 – acetonitrile, 20:80 v/v. Flow rate 1 mL/min. UV detection at 270 nm; α(AX) is abscissa label, ordinate label is k’.
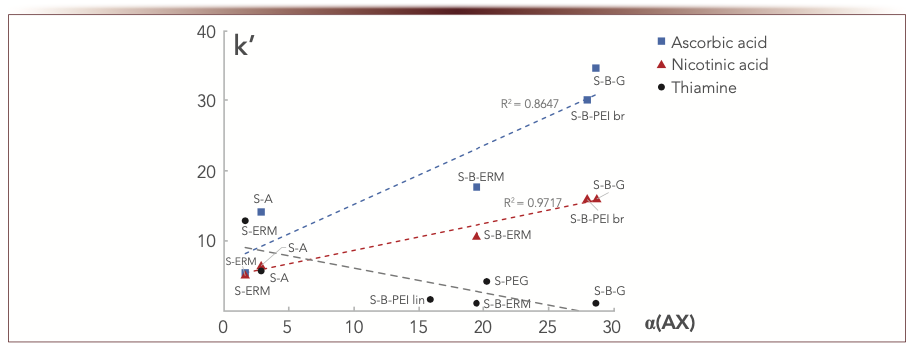
Ascorbic acid demonstrated stronger retention as compared to nicotinic acid on all the phases (Figure 2). This is consistent with its higher hydrophilicity, and thus reflects larger partitioning impact into its retention mechanism. The latter is supported by the results previously discussed in (1), where nicotinic acid showed the predominant impact of the adsorption mechanism in its retention, whereas partitioning balanced adsorption for ascorbic acid for all the described adsorbents.
For complex samples containing analytes of different charge, it is useful to evaluate the conditions for determination of both negatively and positively charged ones. For this purpose, the mixture of water-soluble vitamins was used which included positively charged thiamine and the acids mentioned above.
Cation-exchange selectivity α(CX) is defined in Tanaka test as N,N,N-trimethylphenylammonium chloride (TMPAC) capacity factor divided by k(U) to account the impact of hydrophilic interactions. Since all columns possess anion-exchange selectivity, positively charged analytes are prone to repulsion effects. So, hydrophobic TMPAC had no retention on the majority of the columns and only S-ERM provided cation-exchange selectivity α(CX) > 1 (Table II). Obviously, for hydrophilic positively charged compounds, the repulsion is compensated with hydrophilic interactions. For thiamine (B1), as such a cation example, the retention response on α(AX) of the phases is shown in Figure 2. B1 retention factors decreasing with the increase of α(AX) obviously confirms the key role of electrostatic repulsion from positively charged groups in the functional layers. Summarizing all the trends for charged analytes from Figure 2, it seems possible to make a choice of the column relying on its α(AX) value, which provides reasonable retention and better selectivity for separation of both positively and negatively charged polar compounds.
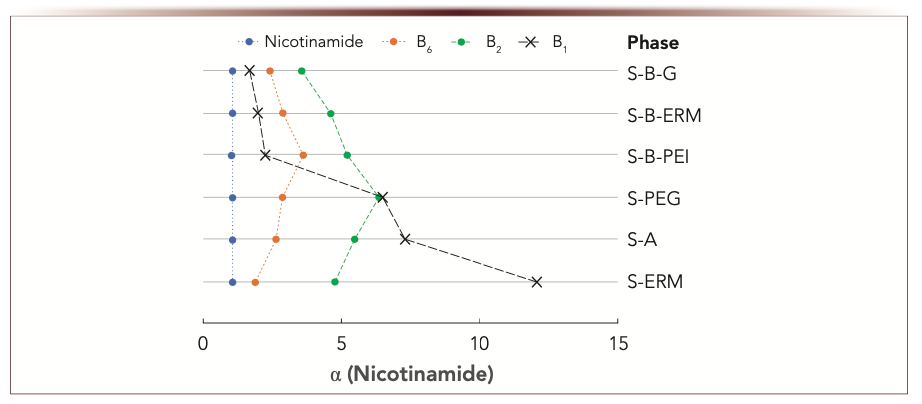
For separation of water-soluble vitamins, ammonium acetate buffer was used in mobile phase according to (2). Its solubility in water-acetonitrile media allows the use of gradient mode and increases concentration to elute strongly retained acidic vitamins from the phases with high anion-exchange selectivity. Selectivity comparison for weakly retained water-soluble vitamins showed more similarities for the phases containing 1,4-BDDGE as the linker and more significant differences for other adsorbents (Figure 3). For the phases prepared without the linker, thiamine demonstrated notably higher retention, which resulted in its elution after riboflavin (B2). The highest selectivity for B1 referred to nicotinamide was demonstrated by S-ERM phase. Using 1,4-BDDGE as a linker resulted in high anion-exchange selectivity of the respective phases (Table II) and repulsion of positively charged B1 with its low retention on S-B-PEI, S-B-G, and S-B-ERM phases. Its highest retention was observed for S-ERM phase where eremomycin (ERM) macromolecule could not fully shield the silica substrate, which is seen from cation-exchange selectivity α(CX) > 1. In this case, dissociated silanol groups are available for electrostatic interactions and attraction of B1.
FIGURE 3: Selectivity plots for weakly retained vitamins. Mobile phase: 100 mM ammonium acetate buffer, wpH 5.8 – acetonitrile, 10:90 v/v. Flow rate 1 mL/min. UV detection at 270 nm; α(Nicotinamide) is abscissa label, ordinate label is phase type.
OH-Selectivity
To compare the chromatographic characteristics of the presented homemade columns with commercially available ones (7,8), α(OH) values for all columns were plotted against respective k(U) values (Figure 4a). S-B-PEI provided much stronger hydrophilic retention k(U) and a little higher OH-selectivity than Hypersil GOLD HILIC with the same PEI functionality. Polymer-coated phases S-B-PEI and S-PEG demonstrated the highest OH-selectivity and hydrophilic retention k(U) as compared to the majority of commercial columns and also home-made monomeric ones. Their α(OH) > 1.8 matches up with the conclusion made in (9) for polymer-modified stationary phases. That could be due to thicker and more uniform coating and a variety of functional sites and groups provided by polymers as compared to the monomers which resulted in increased adsorbed water layer and thus hydrophilic partitioning property of the phase.
FIGURE 4: Retention–selectivity plots for the set of stationary phases presented in (a) (1–8), and (b) selected home-made phases (1–6). Columns: 1 = Hypersil GOLD HILIC (5 μm), 2 = Hypersil GOLD Silica (5 μm), 3 = Hypersil GOLD Silica (1.9 μm), 4 = Syncronis Silica (5 μm), 5 = Accucore HILIC (2.6 μm), 6 = Acclaim Mixed Mode HILIC-1 (5 μm), 7 = Acclaim HILIC-10 (3 μm), 8 = Acclaim Trinity P1 (3 μm), 9 = ZIC-HILIC (5 μm), 10 = Nucleodur HILIC (3 μm), 11 = Amide-80 (5 μm), 12 = XBridge Amide (3.5 μm), 13 = PolySULFOETHYL (3 μm), 14 = PolyHYDROXYETHYL (3 μm), 15 = CYCLOBOND I (5 μm), 16 = LiChrospher Diol (5 μm), 17= COSMOSIL HILIC (5 μm), 18 = Sugar-D (5 μm), 19 = NH2-MS (5 μm). For Figure 4a, k(U) is abscissa label, ordinate label is α(OH). For Figure 4b, k(U) is abscissa label, ordinate label is α(OH).

Among amide-containing phases, S-A demonstrated the highest OH- selectivity and k(U) close to XBridge Amide, where S-B-ERM, XBridge Amide and Amide-80 columns provided the same α(OH) value. S-A also demon- strated hydrophilic selectivity α(OH) and k(U) retention close to those for Hypersil GOLD HILIC (PEI-type phase [8]) and amino phase NH2-MS. Low OH-selectivity was observed for S-ERM providing good hydrophilic retention k(U) matching up with k(U) for XBridge Amide and NH2-MS also containing amide or amino groups, respectively. S-ERM is the only phase in the set which is based on glycidoxypropyl silica as a substrate (Table I), and it is located in another region of the plot (Figure 4b). For the phases obtained using 3-aminopropyl silica good correlation of OH-selectivity and k(U) was observed.
According to (9), the α(OH) parameter represents the phenomenon of hydrophilic partitioning, and it could be used to estimate the thickness of the adsorbed water layer on the surface of the stationary phase. Based on the data given in (9), 1.0–1.2 nm water layer thickness could be adsorbed on the surface of our amide- and polymer-containing homemade phases. Thus, introducing highly hydrophilic amide groups or polar polymers provided the highest OH-selectivity, whereas polymer-coated phases demonstrated improved hydrophilic retention k(U) among the homemade and commercially available columns.
For the homemade set of adsorbents, the selectivity for the analytes differing in OH-group in their structures was compared to α(OH) values obtained from the Tanaka test. For this purpose, we used the pairs of amino acids: Tyr/Phe and Ser/Ala. The mobile phase for amino acids separation required potassium phosphate buffer solution, wpH 6.5 and acetonitrile, 15:85 v/v. As it is presented in Table II, α(Tyr/Phe) > α(Ser/Ala) and these pairs of acids had similar trends but no full correlation with α(OH) of the adsorbents when different from Tanaka test conditions were used. However, the highest OH-selectivity for amino acids was obtained with polymer-coated phases characterized by high α(OH). Remarkably, for S-A phase the reversed elution order was observed for Ser/ Ala when less hydrophilic amino acid retained stronger despite the moderate α(CH2) for this phase. Some specific interactions probably define higher retention of alanine on S-A phase.
Efficiency
It was shown in (3) that, when comparing efficiency for branched and linear polymers in the functional layer, the highest values were obtained for the linear ones, both PEG and PEI. In (1), one can see that using 1,4-BDDGE as a linker resulted in lower efficiency for S-B-A as compared to S-A. The same linker introduced in stationary phase containing ERM provided significantly higher efficiency for S-B-ERM as compared to S-ERM (5). Therefore, the way of attaching ERM with spacing of functional groups resulted in high efficiency for the phases with macromolecules in the functional layer (up to 5 times higher N/m for S-B-ERM as compared to S-ERM). The reason could be better shielding of the silica substrate with 1,4-BDDGE because three linkers can be attached per each amino group on its surface.
Among all phases discussed here, the highest efficiency (35,000–45,000 N/m) for all the tested classes of analytes was demonstrated by S-A obtained with monomeric amide ligands via the Ugi reaction. The lower range of 15,000–40,000 N/m was provided with phases modified with macromolecules, namely, linear polymers (both PEI and PEG) and eremomycin attached via the linker. The phases containing branched PEI and PVP demonstrated efficiencies below 20,000 N/m, probably due to conformational mobility of such functional layers and mass transfer limitations (3).
Through our examples, it could be concluded that using linear polymers for creating functional layers is advantageous for preparing high efficiency columns as compared to branched ones. Also, attaching ERM macromolecule with the branched linker 1,4-BDDGE allowed to obtain efficient phase S-B-ERM for vitamins separation. While creating amide functionality resulted in the highest efficiency for all the tested classes of compounds for S-A phase.
Separation Abilities
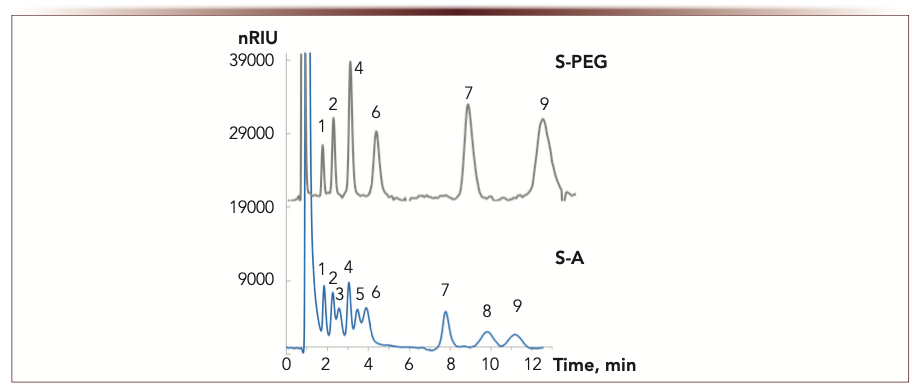
For sugars where hydrogen bonding has significant effect on the retention, polymers in functional layer (such as PEG) provided the highest retention factors (Figure 1). Despite this advantage, separation efficiency for sugars was lower in case of polymer coatings as compared to those modified with monomers. The best separation of sugars was achieved on amide phase S-A (Figure 6), which provided resolution of maltose and lactose having the same log P value (-5.03, calculated via Epiweb 4.1) with the highest efficiency (up to 35,000 N/m, Figure 5).
FIGURE 5: Efficiency obtained for the packed adsorbents (100 × 3 mm column i.d., particle size 5 μm). The separation conditions for each class of analytes are given in Figures 1–3 (1–6). Packing conditions given in references (1–5) were optimized and reproducible. Compound type is abscissa label, ordinate label is N/m.
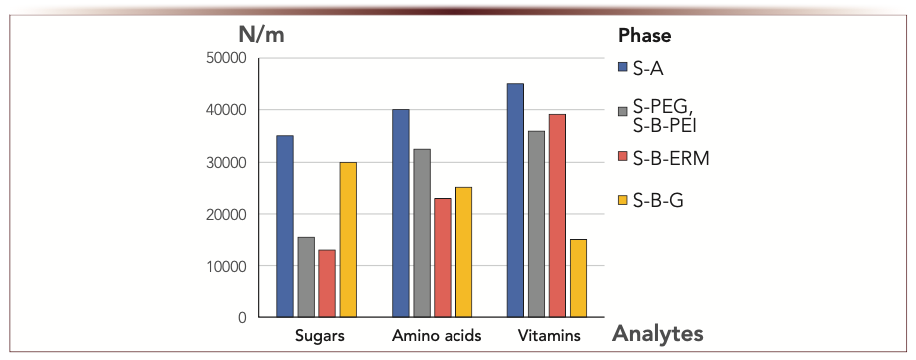
FIGURE 6: Chromatograms for sugars (10–100 ppm). Mobile phase: deionized water:acetonitrile, 15:85 v/v. Detection: RID. Flow rate 1 mL/min. Columns: 100 × 3 mm i.d. 1 = ribose, 2 = xylose, 3 = arabinose, 4 = fructose, 5 = mannose, 6 = glucose, 7 = sucrose, 8 = maltose, 9 = lactose. Retention time (min) is the abscissa label, the ordinate label is nRIU.
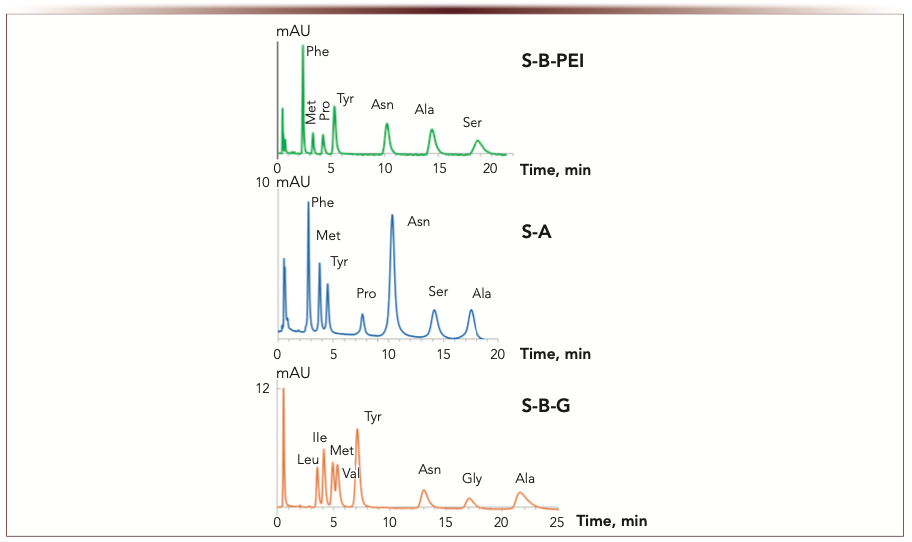
Polymer-coated phases also demonstrated high selectivity for amino acids. S-PEG phase provided the maximum k’ values for amino acids, but required about 40 min for separation. S-B-PEI or S-A provides faster separation with higher efficiency and Tyr/Pro resolution (Figure 7). Zwitterionic S-B-G is also a good choice for amino acid analysis. Its unique feature is to separate Leu, Ile, and Val in the same run with other amino acids.
FIGURE 7: Chromatograms for amino acids (10-100 ppm). Mobile phase: phosphate buffer, wpH 6.5; 5 mM for S-PEG, S-B-G; 1 mM for S-A – acetonitrile, 15:85 v/v for S-PEG, S-A; 12:88 v/v for S-B-G. Flow rate: 1 mL/min. Columns: 100 × 3 mm i.d. UV detection at 210 nm. Retention time (min) is the abscissa label, the ordinate label is mAU. Model mixture including Leu, Ile, Val, and Gly was used for S-B-G to demonstrate its selectivity advantages.
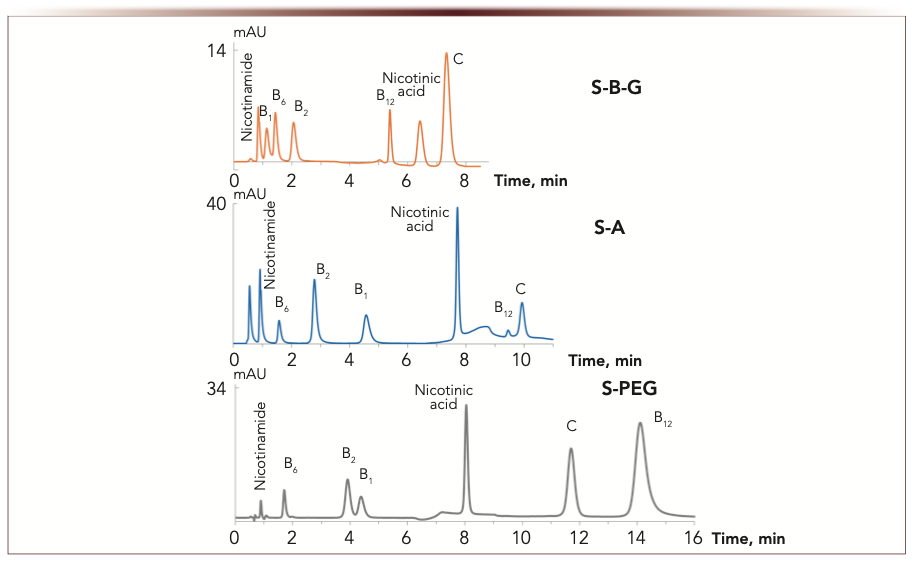
The best resolution of water-soluble vitamins was provided with phase S-A. Polymer-coated S-PEG demonstrated some limitations in selectivity for weakly retained B1/B2, while S-B-PEI was inferior in both efficiency and selectivity. Introducing the linker into functional layer resulted in decreased resolution for weakly retained vitamins which required low water content in mobile phase for their separation. At the same time, introduction of the linker was advantageous for ERM-coated phases bringing higher efficiency and better separation of water-soluble vitamins and amino acids. As shown in (5), both ERM-coated phases also demonstrated chiral selectivity.
The best separations of polar analytes with various stationary phases are demonstrated in Figures 6–8 (1–5).
FIGURE 8: Chromatograms for water-soluble vitamins (riboflavin – 4 ppm, nicotinamide – 10 ppm, others – 50–200 ppm). Mobile phase: 100 mM ammonium acetate buffer (A) – acetonitrile (B). Gradient elution for S-B-G: pH 6.2, 0–3 min 8% A, 3–5 min 8–30% A, 5–10 min 30% A; for S-A: wpH 5.4, 0–5 min 10% A, 5–8 min 10–28% A, 8–12 min 28% A; for S-PEG: wpH 5.8, 0–5 min 8% A, 5–6 min 8–20% A, 6–16 min 20% A. Flow rate: 1 mL/min. UV detection at 270 nm. Retention time (min) is the abscissa label, the ordinate label is mAU.
Conclusion and Prospects
Tanaka HILIC test represented a good algorithm for general characterization of hydrophilic stationary phases. However, it does not always reflect structural differences in the functional layer for retention and separation of various classes of polar substances. By changing conditions and including analytes providing specific interactions with particular surfaces one can complement column characterization to simplify the prediction of retention and selectivity parameters and find the utility of a given phase in contrast with a standard test result.
From the set of described stationary phases, polymer-coated and amide columns demonstrated the best selectivity and efficiency. Specific hydrophilic interactions with amides of S-A phase provided advanced performance and improved separation of polar analytes as compared to polymer-coated adsorbents. Additional advantage of amide phase is the ease of structure variation resulting from flexibility of the Ugi reaction used for the synthesis (1).
The potential for efficiency improvement for polymer-coated phases could include further modification using hydrophilizing or crosslinking agents. Optimizing the structure of a zwitterion and the way of its attachment to the substrate could bring advantages in performance for zwitterionic adsorbents.
Further development of new phases for HILIC with improved selectivity should be focused on the creation of novel chemistries with diversity of functional groups providing multiple types of interactions. Also, flexible synthetic ways look promising for fine tuning of chromatographic performance of the resulting adsorbents.
References
(1) Chikurova, N.; Shemyakina, A.; Shpigun, O.; Chernobrovkina, A. Multicomponent Ugi Reaction as a Tool for Fast and Easy Preparation of Stationary Phases for Hydrophilic Interaction Liquid Chromatography, Part I: The Influence of Attachment and Spacing of the Functional Ligand Obtained via the Ugi Reaction. J. Chromatogr. A 2022, 1666, 462804. DOI: 10.1016/j.chroma.2022.462804
(2) Chikurova, N.; Shemyakina, A.; Bryskina, D.; Nuriev, V.; Komarov, A.; Statkus, et al. A Novel Adsorbent for Hydrophilic Chromatography Based on Silica Modified by the Ugi Reaction. J. Anal. Chem. 2021, 76 (9), 1083–1092. DOI: 10.1134/S1061934821090033
(3) Popov, A.; Maksimov, G.; Shpigun, O.; Chernobrovkina, A. Adsorbents with a Covalently Bonded Polymer Layer for Hydrophilic Interaction Liquid Chromatography. J. Anal. Chem. 2022, 77 (9), 1173–1183. DOI: 10.1134/S1061934822090106
(4) Popov, A.; Maksimov, G.; Smolenkov, A.; Shpigun, O.; Chernobrovkina, A. Novel Adsorbents for Hydrophilic Chromatography Based on Silica Gel Covalently Modified with Poly(ethylene glycol). Moscow Univ. Chem. Bull. 2021, 76 (1), 75–84. DOI: 10.3103/S0027131421010132
(5) Chikurova, N.; Prosuntsova, D.; Stavrianidi, A.; Staroverov, S.; Ananyeva, I.; Smolenkov, A.; Chernobrovkina, A. Novel Mixed-Mode Adsorbents for HPLC Based on Different Substrates Modified with Eremomycin. J. Anal. Chem. 2023, 78 (5). In press.
(6) Chernobrovkina, A.; Sedov, Y.; Shpigun, O.; Zatirakha, A.; Smolenkov, A.; Kovalenko I. Influence of Zwitterion Nature on Chromatographic Performance of HILIC Stationary Phases. Book of Abstracts, HPLC 2017 2017 (Prague, Czech Republic). p. 129.
(7) Kawachi, Y.; Ikegami, T.; Takubo, H.; Ikegami, Y.; Miyamoto, M.; Tanaka, N. Chromatographic Characterization of Hydrophilic Interaction Liquid Chromatography Stationary Phases: Hydrophilicity, Charge Effects, Structural Selectivity, and Separation Efficiency. J. Chromatogr. A 2011, 1218, 5903–5919. DOI: 10.1016/j.chroma.2011.06.048
(8) Dolci, M. On the Characterization of Hydrophilic Interaction Luid Chromatography Stationary Phases. Trends Chromatogr. 2012, 7, 57–73.
(9) Ikegami, T.; Taniguchi, A.; Okada, T.; Horiec, K.; Arasea, S.; Ikegami, Y. Functionalization Using Polymer or Silane? A Practical Test Method to Characterize Hydrophilic Interaction Chromatography Phases in Terms of their Functionalization Method. J. Chromatogr. A 2021, 1638, 461850. DOI: 10.1016/j.chroma.2020.461850
Alla Chernobrovkina is an associate professor in the Department of Chemistry at Lomonosov Moscow State University, in Moscow, Russia. Direct correspondence to: chernobrovkina@analyt.chem.msu.ru

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.











