Insights into the Shape-Selective Retention Properties of Porous Graphitic Carbon Stationary Phases and Applicability for Polar Compounds
In this article, the ability of porous graphitic carbon (PGC) to discriminate between structurally similar compounds is described. After a brief overview of the particle technology and its use in liquid chromatography (LC), two case studies are presented using small-molecule probes to illustrate the importance on how molecular shape and the ability to interact with the graphitic surface lead to the resolution of structural isomers. Finally, an application of this technology in the area of glycan analysis is showcased where released N-glycans from human immunoglobulin G (IgG) were resolved without derivatization, enabling the glycan profile of a biotherapeutic to be determined.
The separation of highly polar compounds by high performance liquid chromatography (HPLC) is still a challenging task. These separations can be approached using ion pair chromatography (IPC), reversed-phase LC (RPLC) separations with a very low content of an organic modifier, or hydrophilic interaction chromatography (HILIC). IPC has the disadvantages of requiring an additional mobile-phase additive, and that the columns must be dedicated for IPC after the first run. Generally, RPLC with a very polar mobile phase is not considered to be very stable (column dewetting). With HILIC, users often lament the lack of robustness and reproducibility. In addition, all of the abovementioned modes utilize silica-based HPLC stationary phases, which are limited in pH range and temperature stability. These modes also have the potential for secondary interactions from active surface silanol groups.
Another promising approach for the retention and analysis of polar compounds by HPLC is using porous graphitic carbon (PGC) as the stationary phase. Carbon materials have been investigated since the 1970s, but only after improving the synthetic route did a carbon stationary phase with satisfying chromatographic and stability properties come to fruition (1,2). Recently, a new carbon particle was developed that has a smaller particle size (2.7 μm) with a narrower particle-size distribution and improved mechanical stability, leading to improved injection-to-injection reproducibility and enhanced efficiency in ultrahigh-pressure liquid chromatography (UHPLC) (3).
PGC phases are superior in some ways to silica-based stationary phases, thanks to PGC’s stability over the complete pH range (1–14) and under temperatures up to 250 °C. Compared with C18 phases, PGC has some similarities with C18, but for many analytes, increased retention is typically observed. Knox and Ross were the two main pioneers who developed the chromatographic characterization of PGC, and during its early development they attempted to explain its unexpected polar retention behavior (4).
It was originally believed that an entirely carbonaceous stationary phase would behave solely as a super-hydrophobic support, but soon it became clear that this was not the case. PGC showed a much higher affinity to retain polar analytes than the standard C18 support. Hence, the term polar retention effect on graphite (PREG) was coined to explain their observations.
PREG is defined as electronic interactions between polarizable groups of an analyte with the surface of graphite. PREG is hypothesized to stem from some form of charge induction or electron ion pair donating/accepting interactions with the pi cloud of graphite (4).
Ross attempted to quantify the contribution of PREG on PGC retention; however, the work was unsuccessful (4). Nevertheless, the term stuck around, and continued research into the retention mechanisms of PGC by other groups gave some substantiation of the claims within the PREG theory (5,6). Notably, the work by De Matteis and others confirmed, using quantitative structure retention relationships (QSRR) in conjunction with multivariate linear regression analysis, that retention was governed by a hydrophobic factor and two electrostatic descriptors related to polarizability and the lowest unoccupied molecular orbital energy (6). Certainly, a more predictive model is warranted for PGC; however, it is obvious that PGC offers alternative retention properties compared to C18 for polar compounds.
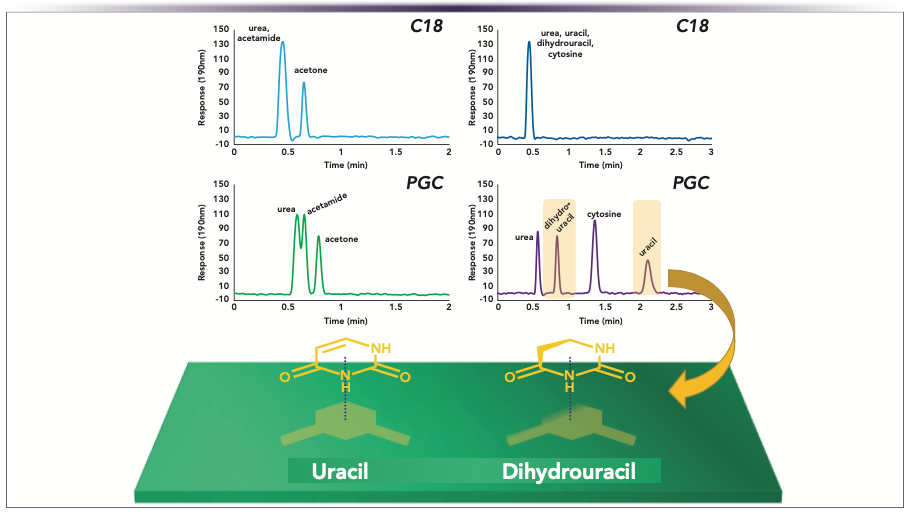
Generally, polar analytes are more retained on PGC than on C18, but how much more firmly depends on the size, structure, and shape of the analyte. Figure 1 demonstrates this concept: Small polar molecules are not well retained on either C18 and PGC, but with polar hydrophilic ring structures, PGC exhibits strong retention, whereas C18 exhibits no retention. The retention difference between uracil and dihydrouracil is quite striking considering their similarities, but the contrast in three-dimensional (3D) structure results in uracil being more strongly retained because of its planar structure.
FIGURE 1: Two different compound mixtures showing corresponding polar retention and peak comparisons on (top) C18 and (bottom) PGC. Chromatographic conditions: columns: Ascentis Express C18 50 x 3 mm i.d., 2.7 μm and Supel Carbon LC 50 x 3 mm i.d., 2.7 μm, mobile phase: 90% water, 10% acetonitrile; flow rate: 0.5 mL/min; temperature: 25 °C, detection: UV-vis 190 nm; sample: 100 μg/mL each; injection volume: 1.0 μL.
Shape Selectivity Case 1
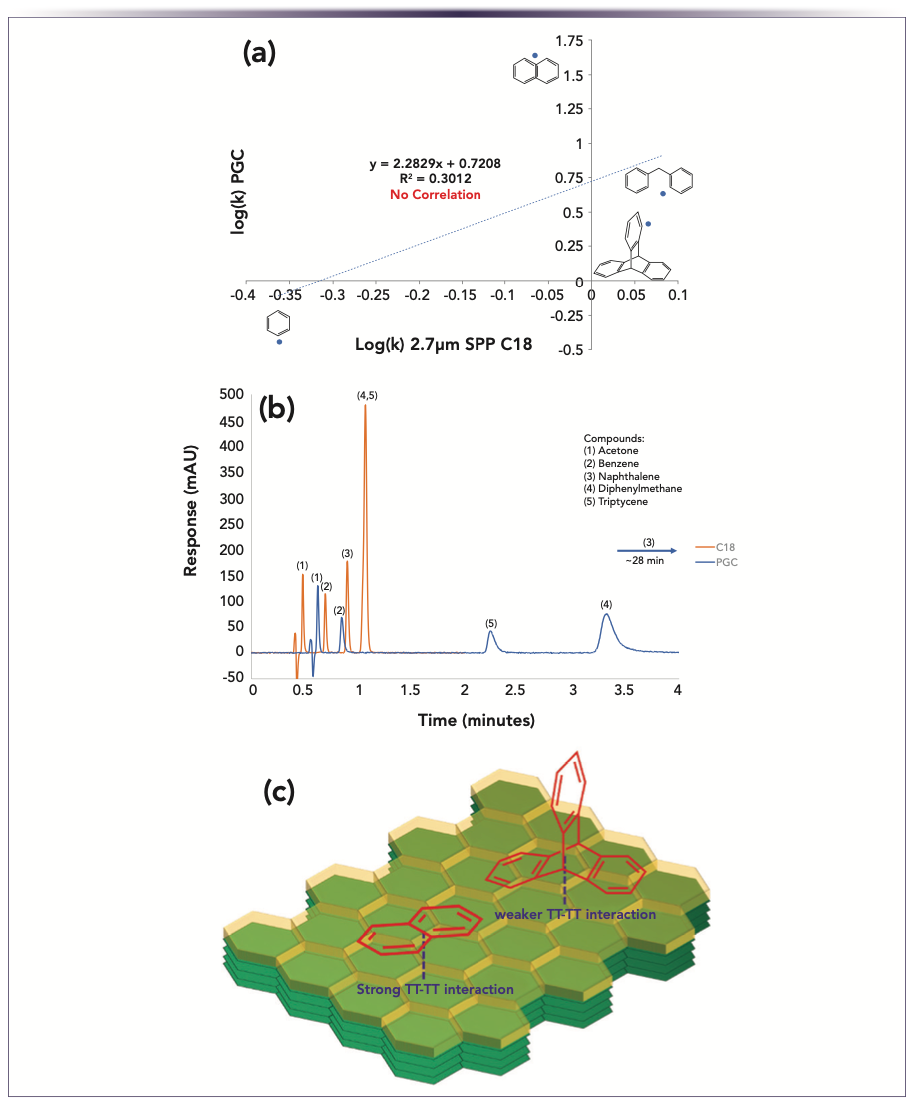
The example shown in Figure 1 alludes to significant analyte molecular shape criteria that are necessary for polar compounds to be adequately retained on PGC. This shape-selective property is not just the case for polar analytes, but for all compounds in general. An instance of this is shown in Figure 2 where a set of hydrophobic analytes (benzene, naphthalene, diphenylmethane, and triptycene) are separated. For the C18 column, broadly speaking, the analytes are eluted in order of their hydrophobicity; however, the PGC column has no similarities in elution pattern to the C18. The reason for these retention changes is best explained by the different shapes of the test compounds and the ability for shape selective interactions to take place with the graphitic surface of the PGC stationary phase. This interaction becomes stronger when there are multiple aromatic systems and the analyte shape or size allows for multiple contact points. In the case of naphthalene, the double ring system results in strong pi–pi interactions with maximal molecular surface area contact with the graphitic surface. On the other hand, triptycene, a much larger and more hydrophobic molecule, is not able to have all of its surface area interact with the stationary phase surface because of its rigid structure.
FIGURE 2: Shape selectivity comparison of PGC and C18. Conditions for (a) and (b) columns: Ascentis Express C18 50 x 3 mm i.d., 2.7 μm and Supel Carbon LC 50 x 3 mm i.d., 2.7 μm, mobile phase: 20% water, 80% acetonitrile, flow rate: 0.43 mL/min, temperature: 25 °C, detection: UV-vis 190 nm, sample: varied concentration each in 20:80 water:acetonitrile, injection volume: 1.0 μL. (c) Illustration of naphthalene’s and triptycene’s pi–pi interactions with a model graphite surface.
Shape Selectivity Case 2: Isomers
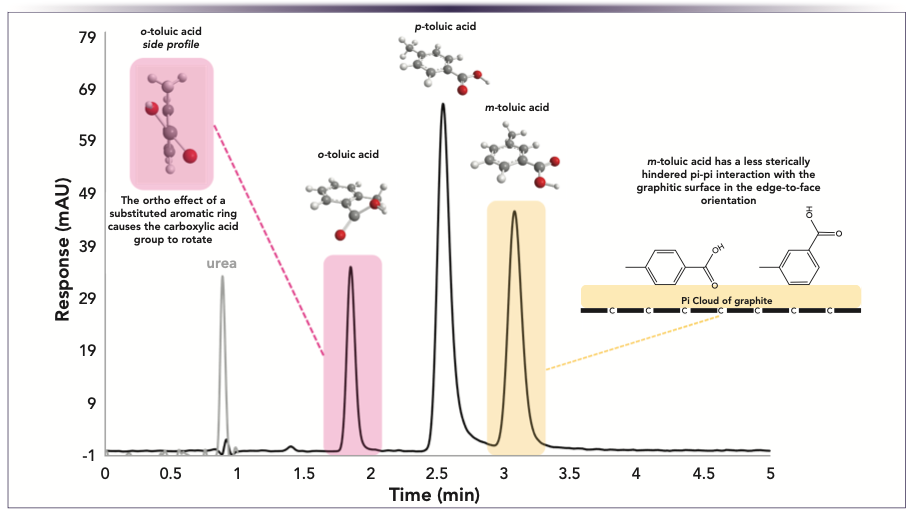
Although the previous examples shows analytes with different amounts of aromatic rings, in this example, all compounds are isomers containing one aromatic ring. Figure 3 shows a separation of toluic acid isomers. For the ring-substituted isomers, the elution order is ortho, para, then meta. Because of the methyl group being ortho to the carboxylic acid group, this forces the carboxylic acid group to twist away from the plane of the benzene ring, resulting in a slightly bent structure. Similar to the previous examples, the ramifications here are a smaller molecular surface area that can overlap with the graphitic plane compared to the meta and para isomers, which are completely flat. Why the para isomer is eluted before meta is not as straightforward. Herein, we must consider other orientations besides face-to-face stacking. In the edge-to-face orientation (molecule is flipped on its edge), the carboxylic acid group slightly hinders the pi–pi interactions, whereas the meta isomer has an orientation that is free from the steric effects of the acid group. These findings are in-line with the molecular modeling studies by De Matteis and others, where the retention behavior of structural isomers was probed in finer detail, highlighting the importance of analyte geometry for pi–pi stacking interactions with PGC (7).
FIGURE 3: Isomer separation using PGC. Column: Supel Carbon LC 50 x 3 mm i.d., 2.7 μm, mobile phase: A: 90% 20 mM ammonium hydrogen carbonate in water, pH 9.0 adjusted with ammonium hydroxide, B: 10% 50:50 acetonitrile:isopropanol, flow rate: 0.3 mL/min, temperature: 50 °C, detection: UV-vis with VWD 230 nm, 190 nm (urea) sample: 50 μg/mL each in mobile phase, injection volume: 1.0 μL.
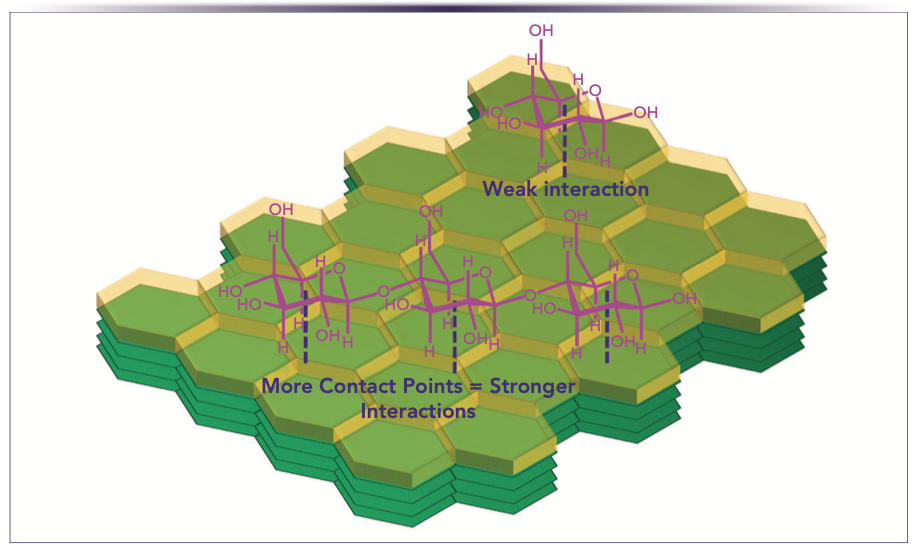
Although the examples shown indicate that PGC is not all-encompassing for polar analytes, PGC still can be a promising option for the analysis of polar compounds, if the target analytes have enough molecular size to interact strongly with the graphitic surface. This observation supports the initial findings on PGC for the analysis of carbohydrates, where PGC lacked retention for monosaccharides, but for disaccharides, oligosaccharides, and larger polysaccharides, acceptable retention times were observed (8). Figure 4 shows this point where a single unit sugar does not have enough size for strong interactions with a model graphite surface, but with multiple sugar subunit molecules, there are more contact points to take advantage of the shape selective properties of the graphitic surface.
FIGURE 4: The importance of multipoint interactions on a model graphite surface.
PGC for Separation of Released Glycans
Glycans are polysaccharides linked by glycosidic bonds. Glycans can be bonded to proteins or lipids. For therapeutic monoclonal antibodies (mAbs), the glycosylation pattern plays a pivotal role in efficacy and lifetime. The glycan profile of a biotherapeutic drug is a critical quality attribute (9).
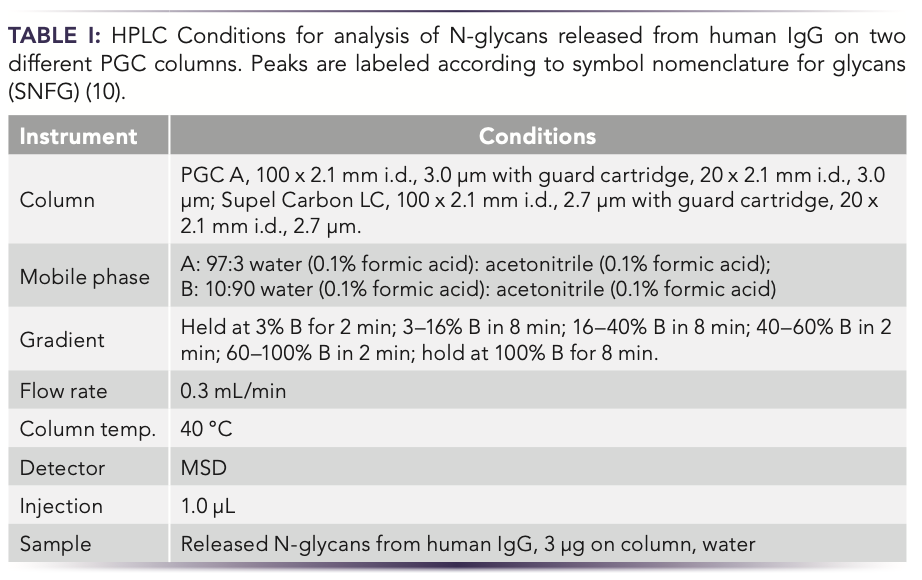
Because glycans consist of different monosaccharides that can be ordered randomly or even branched, numerous glycans exist, and analysis of these polar compounds is challenging. HILIC with derivatization is the method of choice for glycan analysis. As an alternative, PGC can be successfully used to separate glycans released from human immunoglobulin G (IgG) under RPLC conditions without derivatization (Figure 5, Table I). In this case, two commercial PGC columns were compared for this analysis.
FIGURE 5: Separation of released N-glycans from human IgG on two commercial PGC columns, PGC A and PGC B/SupelCarbon LC (data courtesy of Professor Hyun Joo An of Chungnam National University in South Korea).
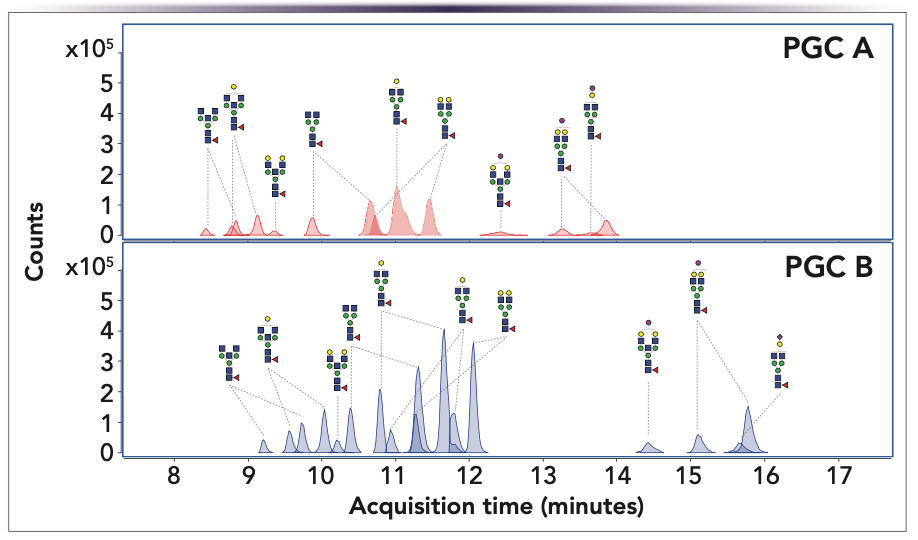
Both columns were able to separate the glycans; however, the performance of PGC B enabled easier identification and accurate mass quantitation. In comparison with the PGC A column, the sensitivity was increased by more than a factor of two, and the separation efficiency was improved, as indicated by the reduction of peak widths by a factor of two. Furthermore, not all glycans were eluted from the PGC A column, which might be the result of too strong of an interaction with the stationary phase. The reasons why PGC B performs remarkably better in this example is an area of further study.
Summary
The examples shown here do not prove or disprove the PREG theory, but they highlight that polar retention on a PGC stationary phase is primarily driven by analyte shape and how molecules can orient themselves with the graphitic surface. These factors should be considered early on in method development when evaluating whether PGC is an appropriate stationary phase for the types of compounds to be separated. Nevertheless, when the unique shape-selective properties of PGC are appropriately identified, PGC stationary phases can be harnessed for fine separations of structurally related compounds, which continue to be a challenge for many practicing chromatographers.
References
(1) Knox, J. H.; Unger, K. K.; Mueller, H. Prospects for Carbon as Packing Material in High-Performance Liquid Chromatography. J. Liq. Chromatogr. 1983, 6, 1–36. DOI: 10.1080/01483918308067647
(2) Knox, J. H.; Kaur, B.; Millward, G. R. Structure and Performance of Porous Graphitic Carbon in Liquid Chromatography. J. Chromatogr. 1986, 352, 3–25. DOI: 10.1016/S0021-9673%2801%2983368-9
(3) Corman, C.; Muraco, C.; Ye, M.; Frantz, C.; Maule, W. Applications Employing New 2.7 μm Porous Graphitic Carbon Particles for U/HPLC. Analytix Reporter 2021, 10, 3–36. https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/ global/documents/264/610/analytix-report-10-br7874en-ms.pdf (accessed 2023-02-08).
(4) Knox, J.; Ross, P. Carbon-Based Packing Materials for Liquid Chromatography: Structure, Performance, and Retention Mechanisms. In Advances in Chromatography, vol. 37; Brown, P. R.; Grushka, E., Eds.; M. Dekker, 1997; pp 73–119.
(5) Lepont, C.; Gunatillaka, A. D.; Poole, C. F. Retention Characteristics of Porous Graphitic Carbon in Reversed-Phase Liquid Chromatography with Methanol–Water Mobile Phases. Analyst 2001, 126, 1318–1325. DOI: 10.1039/B102719K.
(6) De Matteis, C. I.; Simpson, D. A.; Euerby, M. R.; Shaw, P. N.; Barrett, D. A. Chromatographic Retention Behaviour of Monosubstituted Benzene Derivatives on Porous Graphitic Carbon and Octadecyl-Bonded Silica Studied Using Molecular Modelling and Quantitative Structure-Retention Relationships. J. Chromatogr. A. 2012, 1229, 95–106.
(7) De Matteis, C. I.; Simpson, D. A.; Euerby, M. R.; Shaw, P. N.; Barrett, D. A. Chromatographic Retention Behaviour of n-Alkylbenzenes and Pentylbenzene Structural Isomers on Porous Graphitic Carbon and Octadecyl-Bonded Silica Studied Using Molecular Modelling and QSRR. J. Chromatogr. A. 2010, 1217, 6987–6993. DOI: 10.1016/j.chroma.2010.08.023
(8) Koizumi, K. High-Performance Liquid Chromatographic Separation of Carbohydrates on Graphitized Carbon Columns. J. Chromatogr. A. 1996, 720, 119–126. DOI: 10.1016/0021-9673(94)01274-1
(9) Bertozzi, C. R.; Freeze, H. H.; Varki, A.; et al. Glycans in Biotechnology and the Pharmaceutical Industry. In Essentials of Glycobiology, 2nd ed.; Varki, A.; Cummings, R. D.; Esko, J.D., et al., Eds. Cold Spring Harbor Laboratory Press; 2009. Chapter 51.
(10) National Library of Medicine, Symbol Nomenclature for Glycans (SNFG), https://www.ncbi.nlm.nih.gov/glycans/snfg.html (accessed 2023-02-08).
Clinton Corman, Cory Muraco, and Michael Ye are with MilliporeSigma, in Bellefonte, Pennsylvania. Frank Michel is with Merck KGaA, in Darmstadt, Germany. Direct correspondence to: clinton.corman@milliporesigma.com

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.











