Characterizing Antibody-Drug Conjugates for Biotherapeutics with the Light Scattering Toolkit
This article is an overview of the different types of antibody-drug conjugates (ADC) characterization that can be performed with the light scattering toolkit to support the development of biotherapeutics.
This article is an overview of the different types of antibody-drug conjugates (ADC) characterization that can be performed with the light scattering toolkit to support the development of biotherapeutics.
Interest in the use of antibody-drug conjugates (ADCs) as targeted therapeutic agents for the treatment of cancer and other diseases has increased significantly. Treatments can be administered with greater accuracy and sensitivity by targeting disease cells, reducing the dose value and therefore the damage caused to healthy tissues.

Photo Credit: Tony Cordoza/Getty Images
However, ADCs often exhibit increased aggregation propensity because of non-specific interactions arising from attached drug-linker moieties. To ensure the efficacy and safety of these conjugates, the drug-antibody ratio (DAR) must be determined and optimized. The DAR directly affects both the potency and toxicity, as well as having a considerable influence on other properties such as stability and aggregation. Furthermore — as is the case for unmodified biotherapeutics — the development of a viable ADC product requires careful determination of formulation conditions that maintain conformational and colloidal stability. This can be an extensive process because it requires empirical screening of a range of buffer and excipient combinations, often without a prior knowledge of degradation pathways and the compounds that might best prevent them. Therefore there is a growing need for more efficient techniques to accelerate the screening process and ensure that only the most promising compounds are advanced into development stages.
There are a number of techniques that are already widely accepted for characterization of ADCs and other biotherapeutics. DAR is often assessed by mass spectrometry (MS), but characterization relies on uniform ionization efficiencies and recovery of all sample compounds, which is often not the case with heterogeneously modified ADCs. Samples containing particles across a wide size distribution can also pose a problem when performing MS because it is difficult to differentiate between species of significantly differing sizes. UV spectroscopy is an alternative technique that can determine DAR by exploiting the unique absorption properties of the antibody and modifier. By assessing absorption ratios at wavelengths specific to each component, DAR can be calculated from molar extinction coefficients. However, this method does require that the antibody and drug/linker exhibit distinct behaviour in the UV spectrum. For example, the modifier needs to absorb at a wavelength where the antibody does not. The results can also be confounded because of Rayleigh scattering of aggregate in the sample, which appears as an increase in absorption — note that this is more pronounced at shorter wavelengths.
Light scattering detection is an alternative method that can characterize aggregates and the propensity for aggregation, as well as perform advanced characterization such as determining DAR. Dynamic light scattering (DLS) can assess aggregation and unfolding, and multi-angle light scattering (MALS) can give an absolute value of Mw entirely in solution based on fundamental relationships between molar mass and the intensity of light scattered by a molecule. Coupling MALS to a fractionation regime such as size-exclusion chromatography (SEC) or asymmetric field-flow fractionation (AFFF) allows mass determination without the need for conventional calibration as well as characterization of oligomers and aggregates in complex samples.
Formulation Screening by High-Throughput Dynamic Light Scattering
Short and long-term stability must be assessed under different chemical and thermal conditions to ensure integrity of the formulation is maintained. Myriad excipients should be screened to identify conditions that support stability, including surfactants, sugars, salts, antioxidants, and amino acids. High-throughput DLS can be a powerful tool for characterizing the presence and degree of aggregation, thermal and conformational stability, and colloidal stability. DLS directly determines the diffusion coefficient (Dt) by measuring the rate of intensity fluctuations of light scattered by the sample, which can then be used to calculate the hydrodynamic radius (Rh) using the Stokes-Einstein equation:

A more detailed analysis of the fluctuation signals gives a complete size distribution without sample fractionation. Although a low resolution technique, DLS is useful for biotherapeutics characterization because it is very sensitive to small quantities of large aggregates and there is sufficient information in DLS to assess both degree of aggregation and propensity for aggregation via temperature and concentration dependent studies.
Linker-Induced Stability Studied by DLS
In addition to determining general formulation stability of an antibody platform, the impact of chemical modification on overall ADC stability must be assessed. DLS can be used to gauge shifts in sample behaviour caused by the addition of pendant groups to the molecular surface. Figure 1 shows the unfolding and aggregation of two different ADCs when exposed to high temperature conditions as indicated by DLS. Both ADCs consist of the same IgG platform and small-molecule drug (at similar DARs); the only difference is in the composition of the cross-linking agent itself. In this case, ADC2 exhibits decreased unfolding and aggregation temperatures relative to ADC1, suggesting it to be a less desirable modificant. Potential dissociation of reversible aggregates with dilution, sometimes seen in SEC, is not an issue when performing DLS, and the low sample volume required for screening saves on preparation time and resources.

Figure 1: Aggregation pathways of two antibody-drug conjugates ADCs studied by dynamic light scattering (DLS) to demonstrate linker-induced instability.
Size Distribution by FFF–MALS
MALS, which allows direct determination of molar mass, can be particularly useful when coupled with SEC or AFFF where samples can be fractionated for identification and quantitation of resident oligomeric species. The relationship between the intensity of scattered light, molar mass, and weight concentration is well-established and can be represented by the following basic relationship:
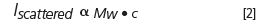
Scattered intensity and concentration are simultaneously determined on-line with a MALS detector and UV or refractive index detector. The calculated molecular weight is independent of the macromolecule's shape. For molecules with a radius exceeding 10 nm, the angular dependence of the scattering intensity can be used to calculate the root mean square (RMS) radius of the molecule. Most ADCs will be below this threshold, and so on-line DLS must be used for accurate sizing.
Fractionation of antibodies and ADCs by SEC is ubiquitous, and coupling SEC to MALS has proven a great boon to biophysical characterization. There are, however, some circumstances where alternative fractionation techniques are required. For example, if quantitation of aggregates typically filtered out by SEC columns is required or when modification results in significant hydrophobic/non-ideal interaction between the ADC and SEC column packing. In these cases, ADCs can be resolved and characterized by field-flow fractionation (FFF). This technique separates particles based on their diffusion in a variable laminar flow and in the absence of a stationary phase.

Figure 2: AFFF elution profiles for an antibody solution. Fractionation conditions can be optimized to (a) detect and quantify aggregate or (b) low-order oligomeric species without the need for multiple columns.
The benefit of FFF is twofold because surface interactions are eliminated and a wide range of oligomers and particles can be resolved without the need for multiple columns and extended separation times. Figure 2 shows fractograms of a monoclonal IgG formulation separated on a single FFF channel by two distinct methods, one optimized for aggregate detection and the other for speciation of low-order oligomers.

Figure 3: Determination of the mAb, drug-linker, and total molar masses of three distinct ADC constructs using SECâMALS.
One of the most promising applications of light scattering to ADC characterization is the calculation of DAR. It can be performed by combining MALS with simultaneous UV absorption and differential refractometry. Similar to DAR determination by differential UV absorbance, this method exploits the distinct UV and dRI behaviour exhibited by the antibody and modifier. As long as UV extinction coefficients and refractive index increments are known for the antibody and modifier (both of which are determined empirically), the molar masses and stoichiometric ratios of each group are established via a simple calculation. Figure 3 shows examples of three ADC constructs, based on the same IgG platform and modified with the different drug-linker compounds; each sample presents a distinct DAR as evaluated. Notably, ADC1 and ADC2 comprise homogeneous DAR across the eluted peak, as evidenced by the horizontal Mw profile (Figure 3[a] and Table 1). In contrast, ADC3 bears a distribution of modifier additions, from ~7 to ~1 (Figure 3[b] and Table 1); such information would not be attainable via bulk assessment by MS or differential UV spectroscopy.
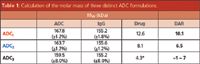
Table 1: Calculation of the molar mass of three distinct ADC formulations.
Conclusion
The light scattering toolkit provides multiple, complementary methods for effective characterization of ADCs and determination of their chemical, thermal, and colloidal stability. The high-throughput capability of DLS is essential for accurately assessing the stability and aggregation of ADCs to evaluate product potential in linker technologies with the greatest efficiency.
The application of MALS to various fractionation regimes allows speciation of samples into subcomponent populations along with molecular weight characterization and quantitation. Most notably, FFF–MALS offers broad-resolution characterization capability for the accurate determination of very small quantities of aggregates as well as a more comprehensive understanding of the oligomeric subcomponents on a single platform.
MALS can also be used alongside UV spectroscopy and refractive index determination to quantify the drug-to-antibody ratio, an important parameter that has considerable influence on the efficacy and safety/toxicity of ADC therapeutics.
As no single technique can independently quantify all of the necessary factors in determining the suitability of a therapeutic agent, light scattering techniques provide a comprehensive characterization platform for the screening of ADCs.
Chris Broomell received a BS in biology from Baylor University, Texas, USA, and a MS in immunology from The University of Texas Southwestern Medical Centre, Texas, USA. He obtained his PhD in biochemistry from the University of California, Santa Barbara, USA, where he investigated structure/property relationships in protein-based biological materials. Broomell completed two years of postdoctoral research in the Center for Bio-Inspired Nanomaterials at Montana State University, Montana, USA, where he worked on the development of novel materials and therapeutic agents based on hierarchical assemblies of virus-like particles. Prior to joining Wyatt Technology he conducted additional postdoctoral research at the University of California, characterizing structure/property relationships in adhesive structures of marine bivalves.
E-mail: info@wyatt.com
Website: www.wyatt.com
This article is from The Column. The full issue can be found here:http://images2.advanstar.com/PixelMags/lctc/digitaledition/October24-2014-uk.html#1

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.













