Fleeting Fashion versus Tried and Tested Techniques
Incognito ponders if fashion is clouding our judgement when it comes to certain tried and tested techniques.
Incognito ponders if fashion is clouding our judgement when it comes to certain tried and tested techniques.
In May 1960, Dr. Lawrence T. Hallet wrote in his editorial introduction to the journal Analytical Chemistry:1
"As individuals our actions are influenced by what is fashionable and acceptable to the majority of those with whom we associate. In many human activities the acceptance of the majority opinion is harmless and contributes to our sense of wellbeing. Similarly, in science, opinion of the majority determines what is fashionable and what are the accepted norms. Very often, this is good and helps science to progress. In some cases, however, it has an adverse effect, as we have determined in reading manuscripts for publication."

Photo Credit: art-4-art/Getty Images
He then went on to discuss how the fashion for using expensive and complex instrumental techniques in a method was often unnecessary, and nothing that a burette and a standardized solution couldn't sort out. It is interesting to consider how things have progressed since then. Hallet was a good man, responsible, alongside one or two others, for the growth in popularity of analytical chemistry, but his attitude in this article was close-minded.
I recently read two articles on advances in the analysis of both intact and digested proteins using non-linear gradients in reversed-phase chromatography. I'd say that non-linear or curvilinear gradients are unfashionable in modern high performance liquid chromatography (HPLC). The last time that I used a non-linear gradient, I selected the gradient profile from a metal plaque on the front of a very old HPLC system and pressed the corresponding numbered button on the pump. This was in the early 1990s, and I'm sure those of you old enough to remember will be smiling at Figure 1 as you remember the gradient profiles that were selectable. Let's take a moment to consider if fashion is causing us to miss out on something powerful.
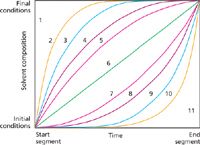
Figure 1: Gradient profiles available on older Waters HPLC systems. Image reproduced from Incognito’s memory.
In complex proteomics separations, peptide peaks resulting from a linear gradient can often be bunched according to some gross physic-chemical property and the "spacing" of the chromatographic peaks is not ideal. In an article published last year, ironically in the journal Analytical Chemistry,2 the development of an algorithm was described that was used to develop a complex non-linear gradient to improve separation of peptides within a mixture. It ensured better selectivity and resolution of chemically similar peptides, which resulted in much better proteome coverage and enhanced confidence in protein identification. Not a straightforward approach, but it did produce measurably better results.
In another article published this year in LCGC North America,3 non-linear, tertiary, gradients were used in the analysis of oxidized and reduced variants of granulocyte colony stimulating factor (GCSF). These analytes also produce chromatograms in which peaks may "bunch" or have widely different hydrophobicity indices that produce large variations in retention time with linear gradients. Concave gradients were used to produce enhanced selectivity in less time than the linear gradient approach. The authors also demonstrated the use of concave gradients to separate charged variants of a monoclonal antibody using a combined pH/ionic strength gradient in ion exchange chromatography. This improved separation of the charged variants, which again tend to "bunch" with traditional linear gradient approaches. The authors went even further to determine the "steepness factor" (n) of a variety of concave gradients and estimated the optimum "n" for both the reversed phase and ion exchange separations, eventually reporting that for a wide range of separations the optimum value of "n" lay between 1.5 and 4.0.
Although complex, all of the above approaches produced significant improvements in the quality of the separations. So why did non-linear gradients fall out of fashion? Surely there are applications outside of biochromatography that would benefit from a non-linear approach? The reasons cited by my colleagues are that concave or convex gradients were replaced by multi-step gradients. They were much easier to accurately reproduce using older pumping equipment and much easier to transfer between instruments with different gradient delivery characteristics.
Does this remain the case with modern equipment?
I would say no. I also think that one of the main reasons we stopped using non-linear gradients is because they were much more difficult to develop and so required more thought to make them robust enough to validate. I would also say that the prevailing fashion in analytical chemistry over the past 20 years has been to make everything as generic as possible to reduce method development time and increase throughput in an attempt to get data out of the door. Did you notice that the previous sentence did not include either the words "expertise" or "quality"?
So, to echo the thoughts of the good Dr. Hallet, I propose that fashion, alongside business requirements and a lack of knowledge of the fundamental principles of separation techniques, may be causing us to be blinkered to possibilities to improve, or even successfully achieve, our separations.
As I write, I can hear my younger self saying "Get a grip Grandad — that's how the world is these days", but just because it's difficult to understand, or it's been done before, or it takes longer to develop or implement, doesn't make it bad.
Core–shell particles were first developed in the 1970s, but we have only recently realized their immense usefulness in routine applications. Upon the reintroduction of these particles (albeit using improved core-to-shell ratios) there was a strong argument against their use because of their limited capacity. I don't hear that argument any longer — it's not fashionable any more.
In the mid 1990s, supercritical fluid chromatography (SFC) was written off when it became clear that reproducibility of gradients using polar modifiers wasn't what we had hoped. However, I see lots of people using preparative SFC with modifiers quite successfully these days. Is fashion clouding our view of what other selectivity or efficiency advantages this technique has to offer? SFC was rejuvenated and became fashionable once more because of its "green" credentials. With just a little development in instrument technology it has been reborn.
To reiterate, just because a method is not straightforward or takes time to develop, that doesn't mean it's not worthy. We shouldn't let fashion cloud our view of techniques, phases, or approaches that have been useful in the past — we may be closing doors that should remain ajar.
References
1. L.T. Hallet, Anal. Chem. 32(6), 575–575 (1960).
2. L. Moruz, P. Pichler, T. Stranzl, K. Mechtler, and L. Käll, Anal. Chem. 85(16), 7777–7785 (2013).
3. V.S. Joshi, V. Kumar, and A.S. Rathore, LCGC North America 32(9), 736–740 (2014).
Contact author: Incognito
E-mail: admin@chromatographyonline.com
This article is from The Column. The full issue can be found here:http://images2.advanstar.com/PixelMags/lctc/digitaledition/October24-2014-uk.html#1

A Final Word from Incognito—The Past, Present, and Future of Chromatography
February 10th 2022After 14 years in print, Incognito’s last article takes a look at what has changed over a career in chromatography, but it predominantly focuses on what the future might hold in terms of theory, technology, and working practices.
Sweating the Small Stuff—Are You Sure You Are Using Your Pipette Properly?
October 7th 2021Most analytical chemists believe their pipetting technique is infallible, but few of us are actually following all of the recommendations within the relevant guidance. Incognito investigates good pipetting practice and busts some of the urban myths behind what is probably the most widely used analytical tool.








