Carbon Nanotubes: Applications in Chromatography and Sample Preparation
LCGC North America
The latest applications for carbon nanotubes in chromatography and sample preparation are outlined.
Carbon nanotubes (CNTs) are cylindrical allotrope nanostructures of carbon with unique structures and unusual properties. The adsorption capacity of CNTs — along with their thermal and chemical stability — make them suitable candidates for new stationary phases, pseudostationary phases, and also for sorbents in solid-phase extraction (SPE) and solid-phase microextraction (SPME). This article outlines the latest applications for CNTs in chromatography and sample preparation.
Carbon nanotubes (CNTs) were officially discovered in 1991 by Sumio Iijima when an arc discharge method, previously used for fullerene synthesis, formed needle-like structures on the negative electrode (1). Microscopy studies revealed concentric tubes formed by two to 50 graphene sheets and these structures were named multi-walled carbon nanotubes (MWCNTs). A few years later, researchers discovered that on the addition of iron (Fe) or cobalt (Co) to one of the electrodes single graphite tubes known as single-walled carbon nanotubes (SWCNTs) were formed (2,3).
The controlled and specific synthesis of SWCNTs and MWCNTs in relatively high amounts is possible by techniques such as arc-discharge (4), laser ablation (5), or chemical vapor deposition (CVD) (6). These processes are, in general, difficult and expensive even before taking purification into account. Cost-effective methods to produce CNTs in larger volumes are still required.
However, the unique properties of CNTs make them potentially very useful in practice. The tensile strength and elastic modulus of CNTs makes them the strongest and hardest materials known because of the sp2 covalent bonds between carbon atoms. But these two properties are only part of their appeal. In general, they show other unique physical, chemical, thermal, and mechanical properties that make them useful for an extensive range of applications, primarily in electronics and electrochemistry. However, they are also used in other fields including gene therapy, drug delivery, neuroengineering, biosensor technology, and biomedical and tissue engineering. As a result, CNTs are probably one of the most intensively studied nanostructured materials (7).
CNTs are also playing a significant role in analytical chemistry (Figure 1). This short review attempts to summarize the most important applications relevant to chromatographers.
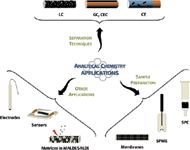
Figure 1: Principal applications of CNTs in analytical chemistry.
Unique Properties of CNTs
Both SWCNTs and MWCNTs have unique physical, chemical, thermal, and mechanical properties. The surface of CNTs can also be easily covalently or non-covalently modified to change their properties. This is useful because many of their desired properties can be preserved, while improving their solubility. Both pristine and modified CNTs have proven to be useful in analytical sciences (8–12) because of the excellent sorption capacity, and great thermal and chemical stability of CNTs. These properties make them potentially useful as stationary phases in gas chromatography (GC), liquid chromatography (LC) and capillary electrochromatography (CEC), as well as pseudo-stationary phases in capillary electrophoresis (CE) (8,9). Additionally, as a result of their sorption capacity — combined with their cave structure and ability to form a wide range of interactions with foreign molecules — they could also be useful stationary phases for sorption extraction techniques, such as solid-phase extraction (SPE) and solid-phase microextraction (SPME) (13,14). CNTs have also been proposed as substrates for matrix-assisted or surface-assisted laser desorption–ionization time-of-flight mass spectrometry (MALDI or SALDI-TOF-MS) (15), and also for the fabrication of polymeric membranes for filtration because of the presence of cores and their ability to let fluids move through them (11).
The electrical properties of CNTs, such as conductivity, dielectric constant, impedance, and refractive index, mean they are sensitive to interaction with analytes, or even with complex biological molecules (8). This is the foundation of their use as gas sensors (16) and biosensors (17). CNTs also have large active surface electrocatalytic properties and good electron transfer to nanoelectrodes.
In this respect, numerous applications describe their use as electrode materials or as modifiers of conventional working electrodes in analytical voltammetry (18). They have also found a place in anodic striping voltammetry because of the combination of electrical properties and the high sorption capacity (19), and as materials to construct and improve electrochemical detectors used in microchip CE (20).
It is important to emphasize that one of the main challenges of using CNTs in analytical chemistry is obtaining pure and well-characterized materials (21). However, commercially available CNTs comprise a distribution of nanotubes of different diameters and lengths depending on the manufacturer, which clearly affects their properties and behavior. For this reason, the selection of the type, dimensions, and producer of CNTs must be carefully chosen to ensure the successful transfer of methods developed between laboratories.
CNTs in Separation Techniques
Chromatographic and Electrochromatographic Applications: As a result of their intrinsic properties, excellent sorbent capacity and thermal and chemical stability, CNTs have been proposed as stationary phases for GC. Non-functionalized pure CNTs are stable up to 1200 °C under inert atmospheres, which prevents column bleeding and enables operation at higher temperatures than conventional columns (22). To produce these GC columns, a thin layer of CNTs is deposited on their surface by CVD (9) producing a homogeneous surface with a small number of polar groups that allows a better peak shape. The possibility of functionalizing the CNTs surface inside the column to improve selectivity has recently been explored; however, it should be noted that thermal stability must be carefully considered because impurities and functionalization directly affect the column reliability (23). Specifically, contaminating high amounts of heavy metals coming from the CNTs synthesis can affect their retention performance. However, the number of applications in this field is still low, and concerns mainly the separation of standard mixtures of volatile compounds such as alkanes, aromatic compounds, esters, and ketones.
The use of CNTs as stationary phases in LC has been explored even less (9,24). They have mainly been used in combination with a second packing material (normally silica) or incorporated in a porous polymeric monolith (11). In nearly all of these works, large columns of 4.6 mm of internal diameter have been used. Figure 2 shows the chromatograms of 11 acidic, neutral and alkaline organic compounds on PS-DVB-CNT columns containing 0%, 1%, and 5% MWCNTs. It should be noted that the resolution and plate number both reduced with superabundant MWCNTs in this specific case (25). Although the applications in this field have increased recently, only test mixtures of analytes have been used in the majority of these works; complex methodologies for real sample analysis have not been developed.
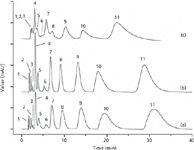
Figure 2: Separation of 11 organic compounds on (a) PS-DVB column, (b) PS-DVB-CNT columns (1% MWCNTs), and (c) PS-DVB-CNT column (5% MWCNTs). Column: PS-DVB and PS-DVB-CNT particles packed in a 150 mm à 4.6 mm stainless steel column. Mobile phase: methanol/water (90:10, v/v). Detection: UV absorbance at 254 nm. Flow rate: 1.0 mL/min. Peak identification: 1 = resorcinol, 2 = sulfadimidine, 3 = benzylalcohol, 4 = aniline, 5 = p-toluidine, 6 = 2-naphthol, 7 = p-methoxybenzaldehyde, 8 = anisole, 9 = N,N-dimethylaniline, 10 = 1,3,5-trimethylbenzene, 11 = 2-methoxynaphthalene. Adapted from reference 25 with permission from Elsevier.
CNTs can be covalently or noncovalently immobilized onto the walls of silica capillaries to perform open-tubular CEC (OT-CEC) to determine different compounds (11). In one example, CNTs have been incorporated in porous polymer monolithic capillary columns to enhance separation of small molecules (26). To the best of our knowledge, CEC with CNT-packed capillaries has not been developed until now. It is also important to mention that there have been applications with CNTs in chips to perform chip-CEC for the determination of alkanes, thioamides, DNA fragments, and colorings (11).
In general, few works have been reported in the chromatographic and electrochromatographic fields so far, but the number of papers published in the future is likely to increase because of the interesting results that CNTs have produced.
Electrophoretic Applications: The use of CNTs in CE lies in their addition to the background electrolytes (BGEs) as pseudostationary phases for the development of electrokinetic chromatography (EKC) (22). However, because raw CNTs are not soluble in aqueous buffers or common solvents, they are usually covalently or non-covalently functionalized to form stable suspensions. In this regard, both MWCNTs and SWCNTs have been oxidized, mixed with surfactants, ionic liquids (ILs), or even dispersed in a microemulsion (aqueous solutions containing dispersed nanometer-size droplets of an immiscible liquid which are surrounded with a surfactant to reduce the surface tension between the two liquid layers) to analyze, for example, drugs in urine, vitamins in pharmaceutical formulations, antioxidants in different matrices, and nucleotides in an extract of chicken (11).
Although several strategies have been adopted to improve solubilization of CNTs in the BGE, it is worth mentioning that it is difficult to obtain accurate and reproducible concentration of the CNTs in the BGE.
CNTs in Sample Preparation
Solid-Phase Extraction: From a detailed review of the current literature one can deduce that CNTs present an adequate sorption capacity for the extraction of both organic and inorganic compounds. In addition, the change in selectivity introduced by the covalent or non-covalent functionalization of their surface has favored their extensive use in this field (11,13,14,27).
On the one hand, the interaction of CNTs with metal ions is believed to occur because of the presence of functional groups in the surface of the material (28) and, as a whole, the metal-removal capability of CNTs is enhanced by the presence of active sites on their surface, the inner cavities and inter-nanotube space. Additionally, the effect of pH is crucial and should be carefully studied. The existence of a pH value called "point of zero charge" (29) or "isoelectric point" (at which the electrical charge density on the CNTs surface is zero) causes the surface of CNTs to become negatively charged at a pH higher than that. This improves the adsorption of cationic species. On the contrary, when the pH is lower than this value, protons compete with metal cations for the same sites on the surface of CNTs. Precisely for this reason solutions containing acidic additives are frequently used to promote the elution of metals.
A number of approaches for metal ion extraction have been focused on the use of oxidized CNTs (o-MWCNTs or o-SWCNTs), obtained by submitting CNTs to a strong acid media at high temperatures. These conditions introduce –COOH, –OH, and –CO– groups, after which different substitution reactions could be performed. In this way, organic species or even biomolecules have also been immobilized on their surface to extend their application (14,27). Complexation of metal ions may not be necessary when using modified CNTs, contrary to what is needed when CNTs are not modified (as it usually occurs with other sorbent materials). In this field, the vast majority of works have been developed to extract inorganic analytes from aqueous samples, and only a few reports have dealt with solid or semisolid matrices (11,13,27).
The strong sorptive interaction of organic analytes has been attributed to π-π electron donor-acceptor relations between the aromatic system of organic molecules (electron acceptors) and the highly-polarizable graphene sheets of CNTs (electron donors) (8). For this reason, a number of authors have worked with non-modified CNTs in this respect, mainly to extract pesticides (13,30), but also other substances such as PAHs, parabens, antioxidants, pharmaceuticals, phthalate esters, phenolic, and biological compounds (11,13).
Conversely, the retention of polar organic analytes has been described to be more efficient with functionalized or modified CNTs. This is because the interaction between functional groups or immobilized organic molecules on the surface of CNTs is more selective. In this respect, o-CNTs have been successfully assayed, together with magnetic CNTs (m-CNTs) (31,32), constituted by the assembly of magnetic nanoparticles onto CNTs via chemical/physical modifications. Figure 3 shows the scanning electron microscopy (SEM) images of Fe/MWCNTs composites with different initial mass ratio of ferrocene to CNTs (33). Magnetic CNTs have facilitated the manipulation of CNTs in dSPE as they can be handled with an external magnetic field provided by a strong magnet.
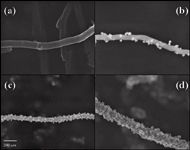
Figure 3: SEM images of (a) pristine MWCNTs and Fe/MWCNTs composites with an initial mass ratio of ferrocene to CNTs of (b) 1:1, (c) 2:1, and (d) 3:1. Adapted from reference (33) with permission from Elsevier.
Organic molecule modified-CNTs have been described for certain applications, and the introduction of molecularly imprinted polymers (MIPs) onto the surface of CNTs has also been exploited as one of the most selective ways to perform SPE, although multi-residue analysis cannot be undertaken often because of the high specificity of the template molecule. As occurred with inorganic analytes, CNTs have been mainly applied to extract organic compounds from different types of water, although different complex matrices (milk, oil, honey, eggs, fish, meat, cosmetics, and soils) have also been analyzed (11,13,27).
CNTs with different dimensions, number of walls, or type of functionalization have been compared. The performance of CNTs in relation to other types of stationary phases, such as C18, activated carbon, graphitized carbon black (GCB), and polymeric sorbents has also been studied. In most cases, CNTs exhibited higher sorption capability or, at the very least, similar effectiveness. With regards to the amount of CNTs required for extraction, it is very frequent to use between 30 mg and 100 mg, even for the extraction of high volumes of liquid samples or extracts (11).
Finally, and like other SPE sorbents, CNTs have been a part of studies regarding automated and miniaturized procedures. Automation also implies a certain degree of miniaturization because SPE mini-columns are used. However, in the case of CNTs and as a result of their dimension and structure, they tend to create flow resistance when using them as mini-column packing materials, with a risk of canalization. For this reason, the use of alternative µ-SPE systems by packing CNTs in the inside of porous materials has offered a good alternative for µ-SPE using mini-columns (11).
Solid-Phase Microextraction: The most challenging task in CNTs-SPME relies on finding a suitable, replicable, and durable coating procedure. Different methods have been settled to deposit CNTs in SPME fibers including sol-gel technology, chemical bonding, electrochemical polymerization, electrophoretic deposition, physical agglutinate by an organic binder, atom transfer radical polymerization, and magnetron sputtering (34,35). Traditionally, fused silica has been used as an appropriate fiber material because of the presence of silanol groups on the surface. However, the fragile fused-silica fibers are being replaced by metal fibers, like stainless steel wires, to solve this important problem.
In general, sol-gel technology has been most widely used but its inadequate reproducibility has made way for other methods, such as simpler chemical bonding methods (35). Also, electrochemical polymerization could be an alternative, but the lack of bonding between the coating and the substrate generates fibers with low thermal stability and swelling in organic solvents. Likewise, electrophoretic deposition has emerged as an alternative to prepare firmer and more stable CNT-SPME fibers. However, until now, a limited number of researchers have used these methods to coat the SPME fibers with CNTs (11,13,35). Physical agglutination with adhesives also provides fibers with low stability and resistance, with the added inconvenience that glues may block the coating pores or even change extraction selectivity.
As in SPE, the selectivity of the CNTs can be modified by introducing functional groups on their surface for the determination of polar compounds. For this reason, both modified and pristine CNTs (MWCNTs and SWCNTs) have been used as SPME coatings for the extraction of organic analytes like multi-class pesticides, phenols, and drugs among others (11, 35), and to a lesser extent for the extraction of inorganic ion traces (11).
Normally, homemade CNTs-fibers have been compared with commercially available fibers to evaluate their performance in terms of extraction efficiency and durability. In most of the papers, CNTs fibers showed higher sensitivity and selectivity and higher or similar extraction efficiency, as well as better thermal and physical resistance.
Membranes: Traditional polymeric membranes used for water purification and gas separation experience different problems: low selectivity, permeability, and susceptibility to obstruction or fouling. CNTs have been used to solve some of these drawbacks and most manuscripts have focused on the use of different types of membranes with CNTs embedded in a polymer matrix. Although these types of membranes offer high stability and efficiency, low cost, and ease of operation, their fabrication is still under development to prevent aggregation. However, some of them have already been used for different applications with success (11).
Other Applications
Electrodes: There are a large number of applications in the literature based on applications of CNTs as electrode materials or modifiers of conventional working electrodes (8,18,36). Their use is justified by their important advantages in electrochemical measurements such as the large active surface at electrodes of small dimensions, the enhanced electron transfer or the often indicated electrocatalytic properties. Analytical methods based on voltammetric stripping techniques have also benefited from the unique properties of these electrodes such as the stronger adsorption of many organic compounds, the less extreme potential of the resulting stripping responses, and the enhanced electroanalytical signals.
Electrochemical Detectors: The excellent electroanalytical properties of CNTs-modified electrodes have favored their use as electrochemical detection systems coupled to separation techniques because they improve the determination of certain compounds (37). In addition, nano-electrodes and nano-electrode arrays made-up from CNTs can be integrated with microelectronics and microfluidic chips, a fact that extends their application in this area.
Gas Sensors and Biosensors: The use of CNTs in gas sensors is based on changes in the electrical properties (conductivity, dielectric constant, impedance, and refractive index) of these materials induced by charge transfer with gas molecules (8,38). In this way, changes in the resistance of the CNT layer (and thus in the impedance) have been exploited for detection of polar (NH3, CO, O3) and nonpolar (He, Ar, N2, O2, CO2) gases (8). The sorption properties of CNTs have been utilized in piezoelectric detection of volatile analytes like alcohols and inorganic gases (He, Ar, O2, N2) and in surface acoustic wave sensors to detect volatile organic compounds (8,38). Also, thermoelectric properties of SWCNTs have been used for the analysis of gases in contact with them (39). Pristine as well as polymer-modified CNTs have been used to construct gas sensors, which takes several hours to release the analyte at room temperature before they can be reused (38).
CNTs have also been used to construct electrochemical biosensors to detect enzymes, genes, immunological proteins, neurotransmitters, and other redox proteins (37,40,41) as a result of their ability to promote electron transfer in electrochemical reactions. The most favorable procedure is the direct attachment of specific proteins or clinically important biomolecules to CNTs. Different types of electrochemical methods have been used in these sensors, including direct electrochemical detection with amperometry or voltammetry, indirect detection of an oxidation product using enzyme sensors, and detection of conductivity changes using CNT-field effect transistors. The electron transference in the detection process can be directly made in the CNTs, in CNTs-polymer composites, in CNTs-metal nanoparticles, and in other CNTs composites (including biological composites).
Matrices in Laser Desorption–Ionization: The use of CNTs as matrices in laser desorption–ionization overcomes some of the disadvantages that exist when molecules with masses lower than 500 Da are analysed by these methods. Most of the organic matrices are decomposed in low mass interferences which generate a high matrix background that hinders mass spectra interpretation (42). The large surface area of CNTs allows the analytes to be dispersed sufficiently, preventing sample aggregation as well as strong UV laser absorption. Nevertheless, contamination of the source by CNTs flying off from the target has been described and therefore some caution must be taken. To avoid this, an adhesion agent may be added to keep the particles attached to the stainless steel plate. In the few existing applications in this field, different molecules have been analyzed, including peptides, carbohydrates, amino acids, pyrenes, low fatty chains, and other low-mass biomolecules (11).
Conclusions
The combination of structures, dimensions, and topologies make CNTs interesting alternative materials, and so research for their applications in separation science is set to increase.
CNTs as stationary phases in GC and LC and as pseudostationary phases in CE are gaining more attention; however, they have modest potential at the moment. Conversely, their growth in sample preparation applications, especially in SPE, has increased substantially, with a huge number of works published in this area.
The most important drawback is that CNTs may contain some impurities from the synthesis procedure that interfere with the ulterior analysis, but this is something that can be easily solved by acid treatment or solvent wash.
The possibility of simple functionalization, binding of complex foreign molecules and solubilization makes CNTs very inspiring and powerful materials. This greatly expands their application in analytical chemistry and further research in this respect is expected to increase.
Although a wide variety of highly pure CNTs are already commercially available they are relatively expensive. This is probably because of their low demand and the low yields obtained in the synthesis steps. However, a reduction in prices is expected when these materials become more commonly used, particularly in sample preparation. CNTs have a rich and unique history with a lot of potential for the future.
References
(1) S. Iijima, Nature 354(6348), 56–58 (1991).
(2) S. Iijima and T. Ichihashi, Nature 363(6430), 603–605 (1993).
(3) D.S. Bethune, C.H. Klang, M.S. de Vries, G. Gorman, R. Savoy, J. Vasquez, and R. Beyers, Nature 363(6430), 605–607 (1993).
(4) C. Journet, W.K. Matser, P. Bernier, L. Laiseau, S. Lefrant, P. Deniard, R. Lee, and J.E. Fischer, Nature 388(6644), 756–758 (1997).
(5) A. Thess, R. Lee, P. Nikolaev, H. Dai, P. Petit, J. Robert, C. Xu, Y.H. Lee, S.G. Kim, A.G. Rinzler, D.T. Colbert, G.E. Scuseria, D. Tománek, J.E. Fischer, and R.E. Smalley, Science 273(5274), 483–487 (1996).
(6) E. Flahaut, C. Laurent, and A. Peigney, Carbon 43(2), 375–383 (2005).
(7) M. Meyyappan, in Carbon Nanotubes-Science and Applications, M.J. O'Connell, Ed. (Taylor & Francis, Boca Raton, 2004).
(8) M. Trojanowicz, TrAC-Trends Anal. Chem. 25(5), 480–489 (2006).
(9) M. Valcárcel, S. Cárdenas, B.M. Simonet, Y. Moliner-Martínez, and R. Lucena, TrAC-Trends Anal. Chem. 27(1), 34–43 (2008).
(10) K. Scida, P.W. Stege, G. Haby, G.A. Messina, and C.D. García, Anal. Chim. Acta 691(1–2), 6–17 (2011).
(11) A.V. Herrera-Herrera, M.A. González-Curbelo, J. Hernández-Borges, and M.A. Rodríguez-Delgado, Anal. Chim. Acta 734, 1–30 (2012).
(12) B. Pérez-López and A. Merkoci, Microchim. Acta 179(1–2), 1–16 (2012).
(13) L.M. Ravelo-Pérez, A.V. Herrera-Herrera, J. Hernández-Borges, and M.A. Rodríguez-Delgado, J. Chromatogr. A 1217(16), 2618–2641 (2010).
(14) C. Herrero Latorre, J. Álvarez Méndez, J. Barciela García, S. García Martín, and R.M. Peña Crecente, Anal. Chim. Acta 749, 16–35 (2012).
(15) M. Najam-ul-Haq, M. Rainer, Z. Szabó, R. Vallant, C.W. Huck, and G.K. Bonn, J. Biochem. Biophys. Methods 70(2), 319–328 (2007).
(16) C. Li, E.T. Thostenson, and T.-S. Chou, Compos. Sci. Technol. 68(6), 1227–1249 (2008).
(17) G.A. Rivas, M.D. Rubianes, M.C. Rodríguez, N.F. Ferreyra, G.L. Luque, M.L. Pedano, S.A. Miscoria, and C. Parrado, Talanta 74(3), 291–307 (2007).
(18) J. Li, J.E. Koehne, A.M. Cassell, H. Chen, H.T. Ng, Q. Ye, W. Fan, J. Han, and M. Meyyppan, Electroanalysis 17(1), 15–27 (2005).
(19) L. Xiao, G.G. Wildgoose, and R.G. Compton, Anal. Chim. Acta 620(1–2), 44–49 (2008).
(20) G. Chen, Talanta 74(3), 326–332 (2007).
(21) P.-X. Hou, C. Liu, and H.-M. Cheng, Carbon 46(15), 2003–2025 (2008).
(22) M. Valcárcel, B.M. Simonet, S. Cárdenas, and B. Suárez, Anal. Bioanal. Chem. 382(8), 1783–1790 (2005).
(23) A. Speltini, D. Merli, E. Quartarone, and A. Profumo, J. Chromatogr. A, 1217(17), 2918–2924 (2010).
(24) A.-H. Duan, S.-M. Xie, and L.-M Yuan, TrAC-Trends Anal. Chem. 30(3), 484–491 (2011).
(25) Y. Zhong, W. Zhou, P. Zhang, and Y. Zhu, Talanta 82(4), 1439–1447 (2010).
(26) S.D. Chambers, F. Svec, and J.M. Frèchet, J. Chromatogr. A 1218(18), 2546–2552 (2011).
(27) R. Sitko, B. Zawiska, and E. Malicka, TrAC-Trends Anal. Chem. 37, 22–31 (2012).
(28) K. Pyrzynska, TrAC-Trends Anal. Chem. 29(7), 718–727 (2010).
(29) H.P. Boem, Carbon 40(2), 145–149 (2002).
(30) K. Pyrzynska, Chemosphere 83(11), 1407–1413 (2011).
(31) L. Jiang and L. Gao, Chem. Mater. 15(14), 2848–2853 (2003).
(32) Y. Deng, C. Deng, D. Yang, C. Wang, S. Fu, and X. Zhang, Chem. Commun. (44), 5548–5550 (2005).
(33) F. Zhao, H. Duan, W. Wang, and J. Wang, Physica B 407(13), 2495–2499 (2012).
(34) V.F. Samanidou and E.G. Karageorgou, Curr. Org. Chem. 16(14), 1645–1669 (2012).
(35) A. Mehdinia and M.O. Aziz-Zanjani, TrAC-Trends Anal. Chem. 42, 205–215 (2013).
(36) Q. Zhao, Z. Gao, and Q. Zhuang, Electroanalysis 14(23), 1609–1613 (2002).
(37) L. Agüí, P. Yáñez-Sedeño, and J.M. Pingarrón, Anal. Chim. Acta 622(1–2), 11–47 (2008).
(38) L. Dai, P. Soundarrajan, and T. Kim, Pure Appl. Chem. 74(9), 1753–1772 (2002).
(39) G.U. Sumanasekera, B.K. Pradham, C.K.W. Adu, H.E. Romero, H.C. Foley, and P.C. Eklund, Mol. Cryst. Liq. Cryst. 387(1), 31–37 (2002).
(40) C.B. Jacobs, M.J. Peairs, and B.J. Venton, Anal. Chim. Acta 662(2), 105–127 (2010).
(41) S.K. Vashist, D. Zheng, K. Al-Rubeaan, J.H.T. Luong, and F.-S. Sheu, Biotechnol. Adv. 29(2), 169–188 (2011).
(42) A.K. Buryak and T.M. Serdyuk, Prot. Met. Phys. Chem. Surf. 47(7), 911–920 (2011).
María Asensio-Ramos obtained her BS degree in chemistry from the University of La Laguna (ULL) in Tenerife (Canary Islands, Spain) in 2007 and her PhD in chemistry in 2012, having performed a pre-doc stay at the Institute of Chemical Methodologies of the Italian National Research Council (CNR) in Monterotondo (Rome, Italy). She currently has a grant from the General Research Support Service of the ULL (SEGAI) and also collaborates with the Department of Analytical Chemistry, Nutrition and Food Science of the University of La Laguna (ULL) in Tenerife (Canary Islands, Spain). Her research focuses on the development of new methods for the extraction and preconcentration of organic contaminants in environmental and food samples by chromatographic and electromigration techniques, also using CNTs.
Antonio V. Herrera-Herrera obtained his BS degree in chemistry from the University of La Laguna (ULL) in Tenerife (Canary Islands, Spain) in 2007 and recently completed his PhD in chemistry at the same university, having performed a pre-doctoral stay at the Institute of Food Science Research (CIAL) which belongs to the National Research Council of Spain (CSIC) and to the Autonomous University of Madrid (UAM). Currently, he has a grant from the General Research Support Service of the ULL (SEGAI) and also collaborates with the Department of Analytical Chemistry, Nutrition and Food Science of the University of La Laguna (ULL) in Tenerife (Canary Islands, Spain). His research focuses on the development of new methods for the extraction and preconcentration of organic contaminants in environmental and food samples by chromatographic and electromigration techniques, using CNTs as extractant sorbents.
Miguel Ángel Rodríguez-Delgado is full professor of analytical chemistry at the Department of Analytical Chemistry, Nutrition and Food Science of the University of La Laguna (ULL) in Tenerife (Canary Islands, Spain). In 1991 he became associate professor, he received his PhD in chemistry in 1994 and in 1997 he became full professor. His research activities involve the advancement of new methods for the analysis of organic pollutants, as well as food contaminants and residues in environmental and food matrices, including the use of CNTs as stationary phases for SPE applications.
Salvatore Fanali is a senior researcher (Research Director) at Institute of Chemical Methodologies, Italian National Research Council in Monterotondo (Rome, Italy). His research activity is focused mainly on the development of new miniaturized instrumentation and methods including CE, CEC, capillary LC and nano-LC — all hyphenated with MS. The techniques are currently applied to food, environmental, drug and forensic analysis. He also studies preconcentration methods.
Javier Hernández-Borges is associate researcher (Ramón y Cajal Program) at the Department of Analytical Chemistry, Nutrition and Food Science of the University of La Laguna (ULL) in Tenerife (Canary Islands, Spain). In 2005 he received his PhD degree in chemistry from the University of La Laguna, performing several pre-doctoral and a post-doc stay at the Institute of Chemical Methodologies of the Italian National Research Council (CNR) in Monterotondo (Rome, Italy). His research currently focuses on the development of new methods for the extraction and preconcentration of pesticides, antibiotics, and estrogens in environmental and food samples by chromatographic and electromigration techniques, also using CNTs. Direct correspondence to: jhborges@ull.edu.es

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Quantifying Terpenes in Hydrodistilled Cannabis sativa Essential Oil with GC-MS
April 21st 2025A recent study conducted at the University of Georgia, (Athens, Georgia) presented a validated method for quantifying 18 terpenes in Cannabis sativa essential oil, extracted via hydrodistillation. The method, utilizing gas chromatography–mass spectrometry (GC–MS) with selected ion monitoring (SIM), includes using internal standards (n-tridecane and octadecane) for accurate analysis, with key validation parameters—such as specificity, accuracy, precision, and detection limits—thoroughly assessed. LCGC International spoke to Noelle Joy of the University of Georgia, corresponding author of this paper discussing the method, about its creation and benefits it offers the analytical community.