Successfully Translating GC Methods from Helium to Hydrogen Carrier Gas
There are many parameters that need to be considered when considering the use of hydrogen as a carrier. Even so, the benefits to chromatography and money saving make this a worthwhile exercise.
There are many parameters that need to be thought of when considering the use of hydrogen as a carrier gas; however, the benefits to chromatography and money saving make this a worthwhile exercise.
The van Deemter minimum of hydrogen is comparable to that of helium, ensuring no inherent loss of efficiency when translating methods. The van Deemter curve also shows that hydrogen remains close to theoretical maximum efficiency at much higher linear velocities (30–70 cm/s, typically) than helium, allowing high efficiency separations in shorter time frames or with shorter or narrower GC columns.
Hydrogen has approximately the same diffusivity as helium, but is a little less than half as viscous. A 50 m × 0.25 mm column requires a helium pressure of 32.3 psig at 100 °C to obtain a carrier linear velocity of 35 cm/s. At the same pressure, the linear velocity with hydrogen will be 77.1 cm/s and the peak retention time will decrease by a factor of 35/77 — that is, retention times will approximately halve. Therefore, to maintain retention behavior we should halve the applied pressure (actual value required 13.9 psig), which will result in approximately equal linear velocity before and after the change. Most modern GC instruments will calculate the required head pressure to operate at a given linear velocity, provided the gas "type" is updated. In this example, the helium flow rate would be 1.88 mL/min. The inlet pressure required to achieve this flow rate with hydrogen would be 18.8 psig, which would result in a linear velocity of 46.6 cm/s; therefore retention times would reduce by a factor of 35/46.6 = 0.75 when using hydrogen at the same flow rate as helium. It is therefore recommended that linear velocity is used as the descriptor for the carrier gas as this directly translates between gases and instruments and is an absolute, which will give constant retention time when translated.
If a constant linear velocity can be maintained during the temperature program by an electronic pneumatic system, then retention times will not change, but this feature is not available in all pneumatic systems. At constant inlet pressure, hydrogen will cause peaks to be eluted earlier, but their elution temperatures will also be reduced and this can change the relative retentions of peaks with divergent chemical characteristics, such as hydrocarbons compared with polar compounds. The same effects will occur, but to a lesser degree, when comparing hydrogen with helium at a constant column flow rate. For ease of method translation one might use a method translation software tool such as Method Translation Software from Agilent Technologies or a similar tool. This tool allows the translation of not only the carrier gas pressure and flow settings, but can also recommend a temperature program to preserve elution order and resolution. It is recommended that when the column is temperature-programmed then each peak should be re-identified if the carrier gas is changed. It might also be a good idea to optimize the temperature program ramp rate by increasing it to restore, as closely as possible, the elution temperatures that were obtained with the helium carrier.
Very often, when switching from helium to hydrogen, one will want to take advantage of the possibilities of using higher linear velocities, and shorter or narrower columns to shorten analysis times. There are a few simple relationships that help with these translations in terms of preserving the elution temperature of analytes and ensuring that the selectivity of the separation is preserved.
When moving to narrower internal diameter columns one needs to preserve the phase ratio (β) to maintain the elution order of analytes. This can be simply done using equation 1:

where rc is the column radius and df is the film thickness (both in micrometers).
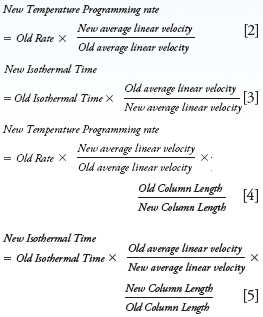
When using a different carrier gas linear velocity or column length the following approximate relationships are useful in estimating the new gradient temperature parameters:
To complete this short primer, it should be noted that when using hydrogen with reduced column dimensions (and higher linear velocity), all system dead volumes should be kept to a minimum and this means paying close attention to column installation and perhaps using a reduced internal diameter inlet liner.
Check with your manufacturer regarding the suitability of hydrogen as a carrier gas with the various detection systems that you use — this is especially important if you are using mass spectrometric detection. All other detector types can be used with hydrogen, but some small modifications to settings or gas supplies and plumbing may be necessary.

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)