Bioinert Versus Biocompatible: The Benefits of Different Column Materials in Liquid Chromatography Separations
Special Issues
In this study, we compare the performance of plastic and metal materials in UHPLC columns designed for the analysis of biological molecules. We evaluate the performance of these materials in terms of inertness, column chromatographic performance, and reproducibility.
For separations of biological molecules, there is concern about potentially irreversible adsorption of analyte molecules onto wetted surfaces in high performance liquid chromatography (HPLC) or ultrahigh-pressure LC (UHPLC) instruments and columns. Solutions to such concerns involve using materials referred to as being bioinert or biocompatible, which traditionally have been made from polyether ether ketone (PEEK). With the emergence of UHPLC, however, materials such as titanium and MP35N alloys are often preferred over PEEK because of their greater ability to withstand high pressures. In this study, we compare the performance of plastic and metal materials for UHPLC column construction. We evaluate the performance of these materials in terms of inertness, column chromatographic performance, and reproducibility to highlight the benefits and drawbacks for biological separations in reversed-phase, size-exclusion, and ion-exchange LC.
Analytical liquid chromatography (LC) methods for protein characterization typically are quantitative. In size-exclusion chromatography (SEC), for example, high-molecular-weight aggregate, or the amount of irreversibly agglomerated protein, is quantitated by percentage of peak area relative to monomer. Because aggregate can potentially be immunogenic, percent monomer is commonly considered a "critical quality attribute" and is monitored throughout the drug development and life-cycle process. Cation-exchange chromatography is also quantitative because it assesses the charge heterogeneity of proteins by analyzing the peak areas of both acidic and basic variants. Understanding the isoelectric point (pI) is especially important for monoclonal antibodies (mAbs) because any changes in the pI will affect clearance and pharmacokinetics of the mAb. Any post-translational modifications (PTMs) to the antibody must be characterized and accounted for, which SEC and cation-exchange chromatography are also used to characterize. In both SEC and cation-exchange chromatography, not only are resolution and selectivity critical to method success, but ensuring that peak areas are consistent is also of critical importance. As such, minimizing nonspecific adsorption of proteins is critical for ensuring quality data and method robustness.

Click here to view full-size graphic
Nonspecific adsorption of proteins is typically addressed by performing several "priming" injections before beginning an analytical LC run. A common practice upon receipt of a new column is to perform consecutive injections, typically 10–100 µg depending on the column dimensions, to adsorb proteins to "active sites" on the chromatographic hardware or media. This involves an inert protein, such as bovine serum albumin (BSA) or other small proteins that are commonly used for blocking steps in other biochemical analytical techniques.
This approach can work reasonably well provided that the protein used for priming injections does indeed cover all the active sites. However, because the mechanism of adsorption is often uncharacterized and poorly understood, a protein such as BSA may not have the same priming effect as the mAb high-molecular-weight aggregate, which often have very unique adsorption characteristics. This possible difference especially becomes a problem with analytical techniques such as SEC because the primary purpose for running SEC is to quantitate the aggregate. If the priming was ineffective, there is a strong possibility that the percentage of monomer is misreported.
Further complicating "priming" is the inconsistency of the mass load required to prime a column. This inconsistency can be observed even when the same batch of chromatographic media is packed into two separate columns. This result strongly indicates that stainless steel surfaces are the primary culprit for nonspecific protein adsorption. Even with the emergence of so-called "bioinert" or "biocompatible" systems, there is still a chance that nonspecific adsorption can occur, thus affecting quantitation and robustness of the analytical method.
In this work, we examine the priming effects observed in reversed-phase, size exclusion, and weak cation-exchange separation modes for columns packed into stainless steel, polyether ether ketone (PEEK), and titanium (Ti) column hardware systems. We also look into other column properties brought about by these different hardware systems, such as column inner diameter reproducibility, column pressure ratings, retention times, and frit flow resistances.
Experimental
All columns were packed in house using the materials described in the discussion section. For comparisons between different hardware systems all columns were packed identically unless otherwise noted.
SEC separations were performed on an Agilent 1100 series high performance liquid chromatography (HPLC) system with a G1329A autosampler, a G1316A column oven, a G1314A ultraviolet (UV) detector, and a G1312A binary pump. The mobile phase was 100 mM sodium phosphate in water. The mobile phase was filtered with a 0.22-µm filter (Phenomenex) before use. The sample was 5-mg/mL γ-globulin and 0.25-mg/mL ovalbumin (Sigma Aldrich) in mobile phase. The samples were filtered with a 0.45-µm syringe filter (Phenomenex) before use. All separations were performed at a flow rate of 0.3 mL/min with a 2-µL injection and detection at 280 nm.
The weak cation-exchange phase with carboxylic functionality was packed into different hardware configurations (150 mm × 4.6 mm), and the protein separations were performed on a Thermo Fisher Scientific ICS 5000 HPLC system with a dual pump, a temperature controlled AS-AP autosampler, a temperature-controlled column compartment, and a variable-wavelength detector. Chromatography and data analysis was controlled by Chromeleon software version 7.2.6.
For the weak cation-exchange separations of proteins, a protein mixture containing 0.5 mg/mL of each cytochrome c (bovine), ribonuclease A (bovine), and lysozyme (chicken egg) in water was used. The injection volume was 7 µL. Mobile-phase A was 20 mM sodium phosphate, pH 6.5, and mobile-hase B was 20 mM sodium phosphate and 1.0 M sodium chloride, pH 6.5. The separations were performed in gradient elution mode where the gradient was 0–100% B in 25 min with 12 min equilibration. The flow rate was 1 mL/min. UV detection was performed at 214 nm.
Monoclonal antibody separations were performed using a Waters H Class bio instrument with a bioquaternary pump, thermostated biosampler manager, thermostated column compartment, photodiode-array detector, and fluorescent detector. The Empower 3 chromatographic data system was used for data collection and analysis. Fluorescence was measured using an excitation wavelength of 280 nm and emission at 360 nm.
Monoclonal antibody separation reproducibility was tested using pH gradients made from a commercial pH-gradient buffer (Thermo Fisher Scientific). Also, 10× buffers (eluent C; pH 5.6) and (eluent D; pH 10.2) were diluted to 1× with purifed water (Millipore) before use. A linear gradient of 0–100% D in 20 min was used. The mAb concentration was 2.5 mg/mL and 3 µL of sample was injected.
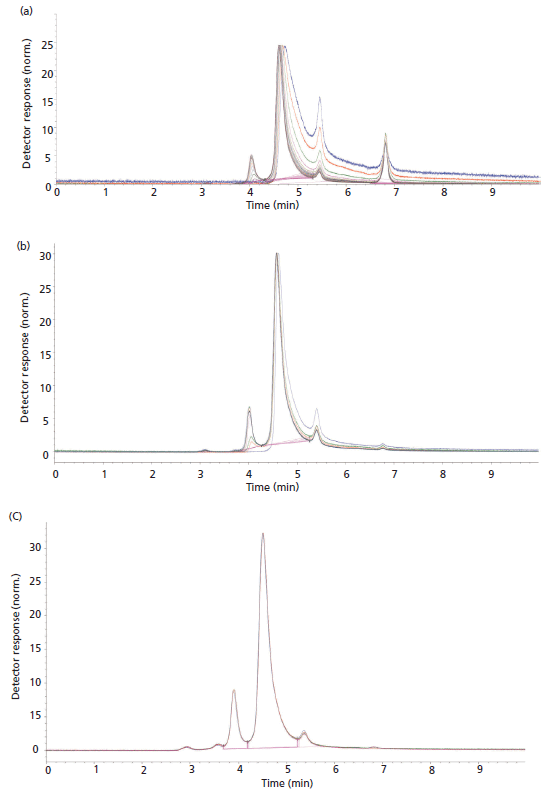
Figure 1: Overlays of injection 1, 5, 10, 15, and 20 are shown for size-exclusion separations of γ-globulin and ovalbumin on (a) a stainless steel column with stainless steel frits, (b) a bio titanium column with bio titanium frits, and (c) a PEEK-lined stainless steel column system with PEEK frits.
Frit permeability studies were performed by measuring the pressure generated when flowing isopropanol through the frit at 40 mL/min. The pressure that was generated by the system tubing and column hardware minus the frit was subtracted from each frit measurement. Column inner diameter measurements were performed using a pin gauge system (Vermont Gauge).

Figure 2: Plots of peak area versus injection number for a SEC separation of γ-globulin and ovalbumin on column packed in PEEK, bio titanium, and stainless steel column hardware.
Reversed-phase separations were performed with columns packed with a 2.6-µm core–shell C18 material. The column internal diameter was measured for each column to ensure they were identical. The columns were each nominally 150 mm × 4.6 mm with the actual measured inner diameter of 4.58 mm. Separations were performed in gradient mode with mobile-phase A being 0.1% formic acid in water and mobile-phase B being 0.1% formic acid in acetonitrile. The gradient was 5–35% B in 10 min. The separations were performed on an Agilent 1260 system with a binary pump, wellplate autosampler, thermostated column compartment, and diode-array detector. UV detection was performed at 214 nm, and the column was thermostated at 40 °C. A 1-µL injection of a sample of 0.15-mg/mL bradykinin, dynorphin A, angiotensin II, Met enkephalin, and Leu enkephalin in 0.1% formic acid in water was used for this analysis.
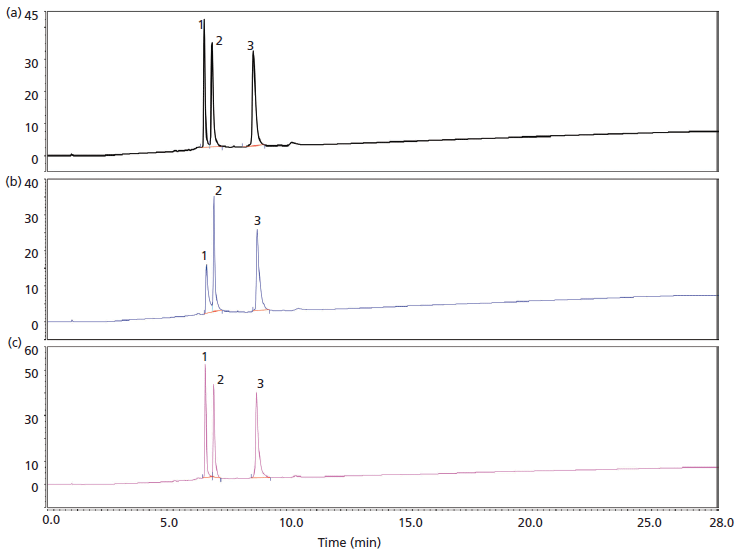
Figure 3: Weak cation-exchange separation of cytochrome c (bovine), ribonuclease A (bovine), and lysozyme (chicken egg) on columns packed in (a) PEEK, (b) stainless steel, and (c) titanium column hardware. Absorbance was measured (UV). Peaks: 1 = cytochrome c, 2 = ribonuclease A, 3 = lysozyme.
Results and Discussion
One of the primary reasons to avoid using non-bioinert materials in chromatography equipment and column hardware is the need to preload, also known as priming, the column or system. Typically, this priming problem manifests itself as increasing peak areas as a function of injection. In some extreme cases, analytes will be totally adsorbed onto the column or system regardless of the amount of material injected. We investigated the number of injections that were required to reach a steady-state peak area in a size-exclusion separation of γ-globulin and ovalbumin. We investigated the same SEC material packed into a stainless steel column with stainless steel frits, a bio titanium column with bio titanium frits, and a PEEK-lined stainless steel column system with PEEK frits. Figure 1 shows overlays of injections 1, 5, 10, 15, and 20 for each of these three columns. Figure 2 shows a plot of peak area versus injection number for these three columns. The stainless steel column took 20 injections to reach a steady state in terms of peak area, while the bio titanium column took five injections to reach maximum peak area. The PEEK-lined column showed a slight decrease in peak area for the first five injections and then reached a steady state. We currently do not have a concrete explanation for this observation, but it has been observed on multiple columns. From the injection overlays shown in Figure 1 there is a definite advantage in terms of column priming for the titanium columns versus that of stainless steel (five injections versus 20 injections).

Adsorption and priming can occur in different separation modes other than just size exclusion. Another important separation mode in the characterization of mAbs is ion-exchange separation. In Figure 3, the separation of a three-protein mixture on a weak cation-exchange mode is shown for a titanium column with titanium frits (Figure 3a), a stainless steel column with stainless steel frits (Figure 3b), and PEEK-lined column with PEEK frits (Figure 3c). We can see in Figure 3 (data is shown in Table I) that the selectivity of the separation is unaffected by the column hardware material. There is, however, a large difference in the peak areas obtained from the same material packed in these different column hardware materials. Table I shows the relative peak area for the chromatograms shown in Figure 3. For the columns packed in stainless steel, the relative total areas are reduced by 54% for cytochrome c (peak 1), 22% for ribonuclease A (peak 2), and 37% for lysozyme (peak 3) when compared to PEEK-lined stainless steel columns. Weak cation-exchange material packed with titanium hardware gave between 87–94% of the peak area that was observed in the PEEK hardware column. The improvements in peak area recovery for the titanium column in comparison to stainless steel also translate to mAb separations. Figure 4 shows injection to injection reproducibility of a mAb separation done on a column packed in titanium hardware.

Because PEEK columns are made via a mold process, the manufacturing process is inherently susceptible to variability in the column dimensions. In Table II, the column internal diameter measurements are shown for 12 different columns made from PEEK, stainless steel, and titanium. The nominal dimension for all columns was 150 mm × 4.6 mm. The column internal diameter relative standard deviation (RSD) for the PEEK columns was 1.31% whereas it was less than 0.5% for both the stainless steel and the titanium hardware. This result will translate into a retention time RSD of 2.6% whereas it is less than 0.6% for the stainless steel and titanium columns. The PEEK manufacturing process also affects the frits. PEEK frits are less permeable at the same filtration rating than their metal counterparts. In Figure 5, the back pressure generated by different frit sizes is shown. The size of the media grade was obtained via a bubble point measurement and was provided to us by the respective frit manufacturers. To obtain the back pressure readings, we attached the frits to an empty HPLC column and subsequently ran isopropanol through them at 40 mL/min using a preparative HPLC pump. We subtracted the back pressure generated by the pump, empty column, and pump tubing at the same flow rate from the readings with the frit, to be able to determine the back pressure just caused by the frit. The high flow rate was chosen to increase the signal and thereby reduce the noise in the measurement. We can see from Figure 5 that the flow resistance of a 0.5-µm PEEK frit was higher than even that of 0.2-µm stainless steel frit. This flow resistance not only adds to the overall back pressure of a packed column, but it leads to problems with fouling when injecting dirty samples or samples with borderline solubility issues.
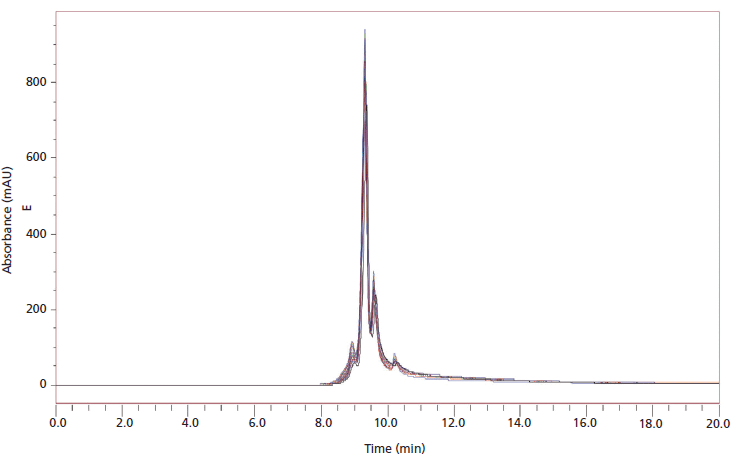
Figure 4: 180 sequential injections of a 2.5-mg/mL monoclonal antibody onto a weak cation-exchange column packed in titanium column hardware.
Figure 6 shows reversed-phase separations of a mixture of five peptides obtained using columns packed in PEEK, stainless steel, and titanium column hardware. The retention times and peak area recoveries were the same on all three columns. The column tubes used for these columns were measured for their inner diameter and tubes with the same inner diameter were chosen. This approach allows us to directly compare the impact of frit permeability on the back pressure generated by these columns during these separations. In Figure 5, it was demonstrated that 0.5-µm PEEK frits gave significantly higher back pressures than their stainless steel counterparts of the same media grade. This high back pressure was also observed in the reversed-phase separation, where the PEEK column generated a back pressure of 227 bar and the stainless steel and titanium columns had back pressures of 209 and 207 bar, respectively.
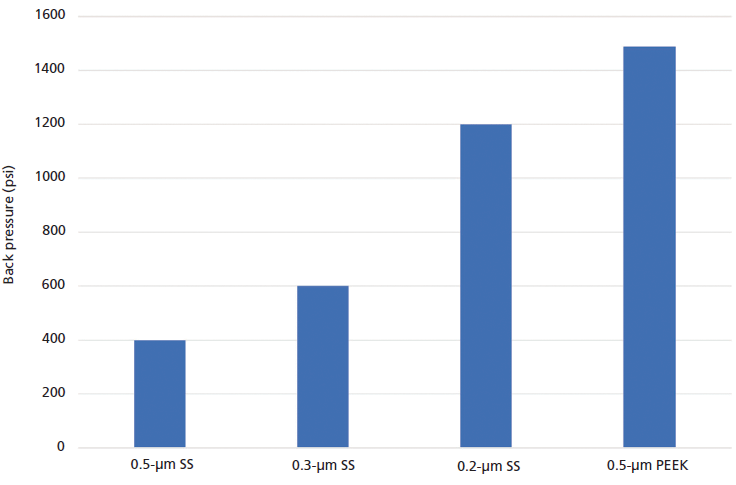
Figure 5: Plot of the back pressure generated by one column frit made of different media filtration grades of stainless steel in comparison to a 0.5-µm media grade PEEK frit at 40 mL/min with 100% isopropanol mobile phase.
Conclusions
Biological samples can exhibit adsorption to traditional HPLC and ultrahigh-pressure liquid chromatography (UHPLC) column hardware, especially when the column hardware is constructed out of stainless steel. This adsorption typically manifests itself via low initial peak areas, requiring several injections to obtain a steady state in the peak area. One alternative to this priming approach is to construct the column using plastic, usually PEEK, materials. However, these materials suffer from lower pressure tolerance, higher column-to-column variation in inner diameter, and higher flow resistance in the frits. In this study, we saw that columns packed in titanium hardware greatly reduced the sample priming and adsorption affects to levels near to, or identical to, PEEK columns. The metal construction of the titanium columns allows for inner diameter reproducibilties, pressure capabilities, and frit flow resistances that are similar to those of traditional stainless steel column hardware. In general, the use of titanium hardware seems to be an excellent compromise between the inertness of PEEK and the mechanical properties of traditional stainless steel.
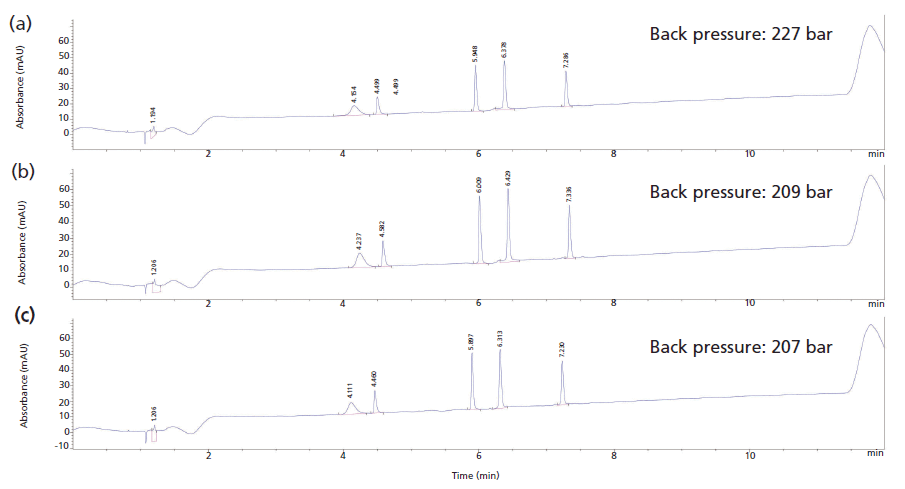
Figure 6: Reversed-phase separation of a mixture of five peptides on columns packed in (a) PEEK, (b) stainless steel, and (c) titanium hardware.
Jason A. Anspach, Srinivasa Rao, and Brian Rivera are with Phenomenex Inc., in Torrance, California. Direct correspondence to: jasona@phenomenex.com

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.











