Avoiding Refractive Index Detector Problems
The refractive index detector can be frustrating to use because of its extreme sensitivity to temperature and other variables. However, it can see things that no other detector can see.
The refractive index detector can be frustrating to use because of its extreme sensitivity to temperature and other variables. However, it can see things that no other detector can see.
The refractive index (RI) detector is unique among common liquid chromatography (LC) detectors because it is truly universal in its detection capabilities. LC detectors based on the absorbance of ultraviolet (UV) light are the most popular detectors because they are simple, reliable, sensitive and respond to a wide range of sample compounds, but only if the analytes have sufficient UV absorbance to detect. Fluorescence detectors are much more selective and can be more sensitive, but compounds must fluoresce to be detected. Mass spectrometry (MS) detectors are increasing in popularity and can provide extremely sensitive and selective detection, but only if the sample can be ionized. The RI detector responds to a universal, bulk property of the analyte — its refractive index. Usually referred to as differential refractive index detectors, these detectors detect peaks based on the difference in refractive index between the analyte and the background mobile phase. This is a benefit that makes the detector universal, but also a problem in that the detector is also sensitive to any other factor that affects refractive index. The major factors are temperature, pressure and mobile-phase composition. This month's instalment describes how RI detectors work and discusses some good practices to follow to get the most out of this powerful detector.
How It Works
Let's first consider how the RI detector works. There are specific design differences between detectors from different manufacturers, but most have the elements of the generic detector shown in Figure 1 in common. All RI detectors depend on the fundamental property of light's refraction, or change of angle, as it passes through different materials. In the case of the RI detector, light passes through the clear walls of the flow cell and through the fluid in the cell. With each transition, refraction takes place and the direction of the light changes slightly. Rather than detect the absolute refractive index (which some detectors can), most detectors measure the differential refraction between a sample flow cell and a static reference cell filled with mobile phase. This, in effect, subtracts the mobile-phase background signal from the sample signal. Because light of longer wavelengths refracts more than shorter wavelengths, a tungsten lamp or light-emitting diode (LED) is used as the light source in most RI detectors. In a quick survey I conducted of commercial RI detectors, various manufacturers used light sources producing wavelengths of 660–880 nm. After the light has passed through the sample and reference cells, it must be detected. Most commonly this is done with a pair of photodiodes. As the refractive index changes, the position of the light beam on the photodiodes shifts so that more or less light shines on each diode. This shift of position can then be detected by comparing the relative intensity of the signal produced by the two photodiodes. In Figure 1, you can see that most of the light strikes the upper diode. With a change in refractive index, the position of the light beam might move down, causing less light to strike the upper diode and more on the lower one.
The basic components of the RI detector shown in Figure 1 are supplemented in real detectors by hardware to stabilize the detector and simplify operation. The reference cell needs to be filled with mobile phase of the same composition as that filling the sample cell (without the analyte, of course). To facilitate this, a switching valve is commonly included to direct mobile phase through the reference cell to refresh or replace the resident liquid. Because it can take several hours for the detector to stabilize, the switching valve may be capable of routing the waste line back into the mobile-phase reservoir to allow the mobile phase to be recycled during warm-up so as to reduce the waste of mobile phase.
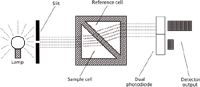
Figure 1: Schematic of a generic refractive index detector, showing the key components.
A change in environmental temperature can be a major problem with RI detectors, because the refractive index of a fluid is dependent on its temperature. For this reason, RI detectors are contained in an insulated compartment. Most commercial detectors can control the temperature above room temperature, typically 30–35 °C up to 50–60 °C, although some models can cool the detector as well. Also, the incoming mobile phase must be at the same temperature as the thermostated portion of the detector, so heat exchangers are included to stabilize the temperature of the mobile phase. Although flow-cell volumes are relatively small, typically 8–10 µL, the heat exchanger volume may be 5–10 times this, or even more. This added volume means that RI detectors usually generate broader peaks than their UV counterparts with smaller total detector volumes.
The inherent design and operating principles of RI detectors leave them susceptible to several problem areas. Specifically, anything that causes changes in the temperature, pressure or mobile-phase composition will create corresponding changes in the refractive index of the mobile phase as it passes through the sample cell. If this is not compensated by the static mobile phase in the reference cell, baseline disturbances will occur. We'll look at each of these problem areas next.
Temperature Problems
As described above, the RI detector is constructed to shield the detector cell from external changes in temperature, both through use of a thermostated cabinet and a heat exchanger for the incoming solvent. However, both of these features are compromises between effectiveness and expense. The cabinet cannot protect against all environmental temperature changes, so it may be necessary to take additional action to protect the instrument from local environmental temperature fluctuations. Although the laboratory temperature control may be quite good, at least as indicated by the thermostat on the wall, the local temperature may vary. Perhaps a heating vent blows hot or cold air directly at the instrument or direct sunlight may cause local warming of the laboratory. In some laboratories, a different temperature is maintained at night than in the daytime. Any of these factors can result in a change in the temperature of the local environment. You may need to block a heater vent or redirect it. In one laboratory I visited recently, the staff had built a cabinet around the LC system to shield it from local temperature fluctuations. It may be necessary to move the instrument to another location with better temperature control.
The heat exchanger's job is to change the temperature of the incoming mobile phase to match that of the solvent in the detector cell. Because the heat exchanger adds extracolumn dead volume to the system, it is a compromise between efficiency of temperature adjustment and minimizing extra volume. To minimize the temperature adjustment requirements, a column oven should be used and set to the same temperature as the detector (or vice versa) so that little or no temperature change is necessary. Also, be sure to insulate the tubing that connects the column to the detector. Some detectors come with an insulated connecting tubing, but a simple homemade insulator can be made by slipping a piece of heavy-walled plastic or rubber tubing over the connecting tubing.
It may take several hours for the detector to warm up and equilibrate with the column temperature, so most RI detectors are equipped with a valve that can divert the waste stream back into the mobile-phase reservoir. In this manner, the mobile phase can be recycled and the system can be left with the flow on for several hours to warm up or left pumping continuously, so it is always ready to use. When samples are run, the valve is switched so that the solvent from the detector is directed to waste. If you do recycle the mobile phase, be sure to replace it once in a while. For a mobile phase that has more than approximately 70% buffer or aqueous component, I recommend changing the mobile phase once a week. When the mobile phase contains at least 30% organic solvent it can be used longer, but it should be replaced every few weeks. Mobile phase that is used for too long can gradually change composition because of evaporation of a more volatile component or may grow bacteria that can block frits in the system. Be sure to replace the reservoir with a clean one instead of refilling the reservoir to prevent passing any contaminants from the previous batch of mobile phase on to the new one.
Temperature-related problems usually show up as baseline drift. Depending on the magnitude of the temperature change and the sensitivity setting on the detector, this may be a gradually sloping or steeply sloping baseline. When baseline drift is a problem, review the preventive steps listed above and see if there is something you can modify to reduce the problem.
Pressure Problems
A second factor that affects refractive index is pressure. For the quietest baselines, the pressure in the flow cell needs to be constant. Most RI flow cells have an upper pressure limit of no more than approximately 100 psi (7 bar), and the use of a back-pressure restrictor after the cell is common. A back-pressure restrictor can be thought of as a spring-loaded check valve that maintains a fixed pressure, such as 75 psi (5 bar), at all times. This will keep the pressure constant and also will keep the system from exceeding the maximum cell pressure. A piece of capillary tubing after the flow cell can also function as a back-pressure restrictor, but the pressure will be related to the flow rate — if the flow rate is inadvertently set too high, a capillary restrictor may cause the permissible cell pressure to be exceeded.
Because LC systems are operated in a constant-flow mode, the pressure should be constant. This is usually the case, but problems with the pumps can cause the pressure to fluctuate sufficiently that the baseline is disturbed, even though other problems such as retention-time shifts are not observed. Pressure problems because of pump malfunctions often will create cycling baselines. To confirm this, you can change the flow rate and the frequency of the baseline cycle should change in accordance to the flow-rate change. For example, a change from 1 mL/min to 2 mL/min should double the frequency of the baseline cycle. Common sources of pressure fluctuations are faulty check valves, leaky pump seals, air bubbles in the pump and more rarely a broken pump piston. The easiest things to check are bubbles in the pump and degassing problems — make sure the degasser is working properly, then purge the pump to release any trapped bubbles and resume operation. Check valve sonication in methanol for a few minutes will often clean a dirty or sticking check valve, or the check valve can be replaced. Pump seal replacement is a little more work, but is something that can be done by following the instructions in the pump service manual.
Mobile-Phase Problems
Any change in the chemical composition of the mobile phase will change its refractive index, as will the presence of dissolved air in the mobile phase. For these reasons, RI detectors are always operated only in the isocratic (not gradient) mode and the mobile phase must be thoroughly degassed. If you have an in-line degasser, as is the case for most LC systems today, be sure to use it. Alternatively, helium sparging is suggested to degas the mobile phase. Because of the extreme sensitivity of the detector to very small changes in refractive index, on-line mixing of the mobile phase will usually create problems. As a result, mobile phases must be hand-mixed so that no change in mobile phase composition occurs within the LC system. It is best to use the mobile phase as the injection solvent so the refractive index change at the column dead-time is minimized.
Remember that the RI detector measures the difference in refractive index between the contents of the sample and reference cells, so the reference cell needs to be purged with fresh mobile phase whenever the mobile phase is changed or replaced with a fresh batch. It is a good idea to purge the reference cell daily to ensure its contents are matched with the mobile phase exiting the column. When changing from one mobile phase to another or washing the mobile phase into a new column, complete equilibration may take longer than you normally allow with UV detection. With UV and most other detectors, allowing 10 column volumes of mobile phase (≈15 mL for a 150 mm × 4.6 mm column) to pass through the column is sufficient for equilibration. It may take longer with the RI detector. Watch the baseline when changing the solvent; drift is common during solvent changeover, so a nondrifting baseline is a good indicator of column equilibration with RI detection. As mentioned above, be sure to change the mobile phase regularly to avoid problems with microbial growth, especially in highly aqueous mobile phases.
Additional Comments
Sometimes RI detectors are used for different applications with either aqueous or nonaqueous solvents. When this is the practice, be sure to flush the entire system (reservoirs, degasser, pump, autosampler and detector) with a series of solvents that are mutually miscible. For example, go from aqueous solvents to 100% acetonitrile or methanol, then to organic solvents. If you are not sure of the history of the system, remove the column and replace it with a piece of capillary tubing. Then flush the entire system with 20–30 mL of isopropanol, which is miscible with both aqueous and organic solvents. Then flush to the desired mobile phase.
Baseline noise can be a critical factor with RI detection. Because RI inherently has poor sensitivity when compared to UV or other detectors, signal-to-noise can be a limiting factor. For this reason, you may want to take advantage of larger detector time constants (noise filters) with RI than with other detectors. A good rule of thumb is to set the detector time constant at 10% of the peak width at baseline or 20% of the half-height width. For example, if the peak is 10 s wide at the baseline, you can use a 1 s time constant. A higher time constant value smoothes the baseline, but too high a value will "smooth" off the top of the peaks, making them broader and shorter.
If you are having a hard time distinguishing the source of a baseline problem between the pump and mobile phase as opposed to a temperaturerelated problem, turn off the pump (or set the flow to 0 mL/min). This will eliminate the pump or mobile-phase problem. If the baseline problem persists, it is because of changing temperature.
Because of its extreme sensitivity to temperature, a byword for RI detection is patience. It will take longer to equilibrate the mobile phase, to warm up the detector or settle down from any system change. For this reason, if time is critical, it is prudent to leave the detector turned on and in a mobile-phase recycle mode. You can reduce the flow rate under these conditions if you desire, but this will leave the system in a standby mode that will return rapidly to normal operation.
If you are looking for alternatives to the RI detector for universal detection, consider evaporative light scattering detection (ELSD) or charged-aerosol detection (CAD). Both of these detectors rely on evaporation of the mobile phase and then detection of the "dust" that is left behind. Both ELSD and CAD can be operated with gradients, which is an additional advantage, but they are restricted to mobile phases that are volatile — so no phosphate buffer is allowed.
And if all else fails . . . read the directions! If you are a normal user of other detectors, such as UV, fluorescence or MS, troubleshooting RI problems may not be second nature. Consult the operation and service manual for your specific detector for troubleshooting and preventive maintenance instructions. If you'd like advice from other users regarding specific problems, consult one of the on-line discussion groups.
John W. Dolan is vice president of LC Resources, Walnut Creek, California, USA. He is also a member of LCGC Europe's editorial advisory board. Direct correspondence about this column should go to "LC Troubleshooting", LCGC Europe, 4A Bridgegate Pavilion, Chester Business Park, Wrexham Road, Chester, CH4 9QH, UK, or e-mail the editor, Alasdair Matheson, at amatheson@advanstar.com

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.












