Application of Silica Monoliths for Bioanalysis
The Column
In this article, the application of silica monoliths with bimodal pore structure (macro and mesopores) is described for the chromatographic analysis of biomolecules including peptides, proteins, and antibodies.
Small peptides can be separated on conventional silica monoliths with mesopore sizes of about 15 nm, but large biomolecules such as antibodies cannot because of an unfavourable mass hindrance. In this article, the application of silica monoliths with bimodal pore structure (macro and mesopores) is described for the chromatographic analysis of biomolecules including peptides, proteins, and antibodies. It is demonstrated that silica monoliths with bigger mesopores of 30 nm can be used for the analysis of antibodies in less than 4 min.
The development of new biological entities (such as peptides, proteins, and antibodies) is increasing because of their potential for targeting drugs in the body. Hundreds of protein-based drugs are already commercially available or close to market introduction because biotechnology processes now enable production of therapeutic biomolecules at reasonable costs.1 Consequently, there is a demand for analytical methods to monitor production processes and quality control of biomolecules for therapeutic purposes. High pressure liquid chromatography (HPLC) is one of the analytical methods that is routinely used for the chromatographic analysis of drugs. At the heart of this method is the HPLC column filled with silica particles2 or silica monoliths.3

(PHOTO CREDIT: REB IMAGES/GETTY IMAGES)
There are a variety of silica particles available; for example, nonporous particles,4–6 totally porous particles,7–8 and more recently, core–shell particles.9–11 Nonporous silica particles are usually prepared with small particle sizes (1.5–2.0-µm), and show high column backpressures and low capacities.
Totally porous particles are available in a broad range of particle sizes (1.5–25-µm) with large surface areas (200–400 m2/g). However, the mesopore size of such particles is in the range of 8–15 nm, which is not ideal for good mass transfer of large biomolecules in and out of the pores. Therefore, silica particles with wider mesopores (up to 30 nm) were developed; and more recently, core–shell particles with mesopore sizes suitable for the chromatography of biomolecules.11
An alternative to classical silica particles are silica monoliths, a single porous silica rod12–17 that can be prepared using a sol-gel process, which allows the independent formation of macro- and mesopores.16–17 Macropores produce high permeabilities that are suitable for big biomolecules because they avoid clogging of columns. The mesopores are prepared by applying a suitable ageing process, which results in high surface areas needed for a sufficient chromatographic separation process.16–17 In this article, the application of silica monoliths with wide pore mesopores of approximately 30 nm for the chromatographic analysis of biomolecules such as peptides, proteins, and antibodies are demonstrated.
Methods and Materials
Samples: A peptide mixture of gly-tyr, val-tyr-val, met enkephalin, leu enkepahalin, and angiotensin II was purchased from Sigma Aldrich. Erbitux (Cetuximab) was a research sample of Merck Serono. Gammanorm IgG was obtained from Octapharma GmbH. All samples were dissolved in water/acetonitrile (90/10; v/v) to which 0.1% TFA was added.
Chromatography: Silica monoliths were prepared according to a sol-gel process.12,16–17 The resulting monoliths possessed a macropore size of either 2 µm or 1.1 µm. The mesopore size was controlled by a thermal process under alkaline conditions and determined to 30 nm. The surface of the silica monoliths was chemically derivatized to obtain either conventional C18e surfaces (long reversed-phase chains) or –CN (cyanopropyl-) surfaces representing short chain C4 reversed-phase materials. All silica monoliths were clad with a solvent and mechanical resistant polymer (polyether ether ketone [PEEK]) and equipped with commercially available endfittings to permit their use as HPLC columns.
Chromatography was performed with an Ultimate 3000 HPLC system (Dionex - Thermo Fisher Scientific GmbH) equipped with an LPG-3400A pump, a WPS-3000SL analytical autosampler, a TCC-3200 column compartment/oven, and a VWD-3400 detector. The columns were 100 × 4.6 mm, either Chromolith HighResolution RP18e with 1.1 µm macropore size and 15 nm mesopore size (EMD Millipore) or silica monoliths with 1.1 or 2.0 um macropore size and 30 nm mesopore size research samples from Merck Millipore, and were operated in gradient mode using acetonitrile/water mixtures to which 0.04% trifluoroacetic acid (TFA) was added. Chromatographic runs were performed either at ambient temperature or 60 °C.
Results and Discussion
The separation of biomolecules using HPLC columns packed with totally porous silica particles is usually associated with the following problems: slow diffusion, limited accessibility of surface for larger molecules, and possible sample conformation changes during elution.18,20 One of the reasons for these problems is the small mesopore size of conventional HPLC packings, usually ranging from 8–15 nm. To overcome these limitations it is advisable to use column materials with wider mesopores of around 30 nm and surface areas of about 150 m2/g.18–20
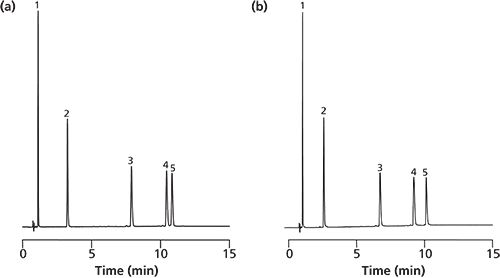
Figure 1: Separation of five peptides on silica monoliths with different mesopore sizes ([a] 15 nm vs. [b] 30 nm). Chromatographic conditions: gradient: acetonitrile 0.04% trifluoroacetic acid/water 0.04% trifluoroacetic acid; 0 min 10/90, 0–10 min 20/80, 10–15 min 20/80; flow: 2 mL/min; detection: 220 nm; injection: 1 µL; temperature: ambient. Samples: 1) Gly-Tyr; 2) Val-Tyr-Val; 3) Met enkephalin; 4) Leu enkephalin; 5) Angiotensin II.
Figure 1(a) shows the chromatographic separation of five peptides performed using a silica monolith with macropore size of 1.1 µm and mesopores of 15 nm. Figure 1(b) shows the separation performed with a silica monolith of the same macropore size but with mesopores of 30 nm. Both columns produced a baseline separation of the peptides with sharp peak symmetries; however, in the case of the silica monolith with 30 nm mesopores the peak pair of angiotensin II and leu enkephalin is separated with a better α-value (α = 1.11 vs. 1.04) in slightly shorter retention times (9.21/10.12min vs. 10.44/10.83 min). This indicates that bigger mesopores are more suitable for the chromatographic separation of oligopeptides.
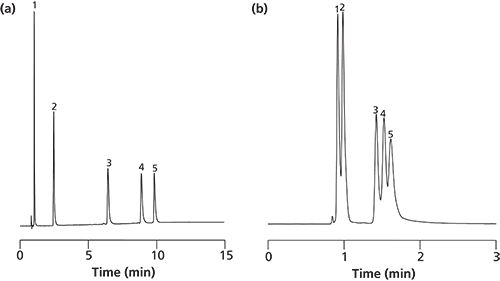
Figure 2: Separation of five peptides on silica monoliths with different surface modifications (RP-18e vs. CN propyl). Columns: Silica monolith (macropore size: 2 µm, mesopore size: 30 nm) with (a) C18e and (b) CN-propyl surface modification. See Figure 1 for chromatographic conditions.
A further issue is the recovery rate, which can be addressed to the mesopore size as well as to its surface modification. A moderate hydrophobicity (C4-reversed phase) may be better than a strong hydrophobicity (C18-reversed phase) for big biomolecules like proteins and antibodies. However, for low-molecular-weight substances (such as the peptides used in Figure 1) the respective selectivity on C18 surfaces is more suitable than the C4 stationary phase. This is shown in Figure 2(a) and 2(b) and reflects the baseline separation of peptides on the silica monolith (macropore size: 2 µm) with mesopores of 30-nm size derivatized with C18 (Figure 2[a]) as compared to the separation on the same silica monolith derivatized with C4 moieties (Figure 2[b]). The latter does not offer a baseline separation of the five peptides, with a total retention time of only 1.6 min for the last peak.
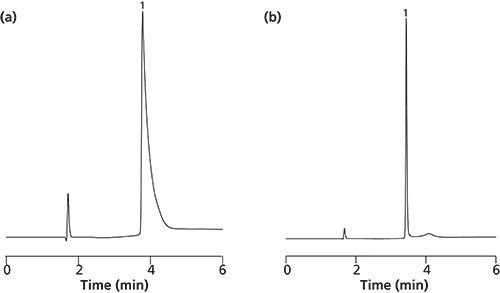
Figure 3: Separation of Erbitux (Cetuximab) on silica monoliths wide-pore (30 nm) with CN-propyl surface modification. Chromatographic conditions: gradient: acetonitrile 0.04% trifluoroacetic acid/water 0.04% trifluoroacetic acid; 0 min 16/84, 0–3 min 60/40, 3–6 min 60/40; flow: 1 mL/min; detection: 220 nm; injection: 1 µL; temperature: (a) ambient and (b) 60 °C.
The biggest challenge in HPLC is the separation of antibodies because they represent the biggest biomolecules with a molecular weight of around 145 kDa. The separation of antibodies is usually accompanied by broad and unsymmetrical peaks that prohibit a quantitative analysis. These problems are related to a hindered mass transfer, limited access to the surface, and possible conformational changes during the elution. They can be overcome by applying silica monoliths with big macro and mesopore sizes (2 µm and 30 nm). Figure 3(a) shows the separation of Erbitux (Cetuximab), a therapeutic antibody against cancer, eluted after 3.8 min at room temperature using a gradient of acetonitrile/water with the addition of 0.04% TFA. Increasing the temperature up to 60 °C (shown in Figure 3[b]) causes only minimal reduction in retention time (3.4 min), but elution of the antibody occurs now as a sharp peak with optimum peak symmetry. This confirms that the application of kinetic energy improves the mass transfer and chromatographic interaction of the antibody with the surface. This result has been confirmed with Gammanorm IgG analyzed at room temperature (shown in Figure 4[a]) and at 60 °C (shown in Figure 4[b]) on the same monolithic HPLC column.
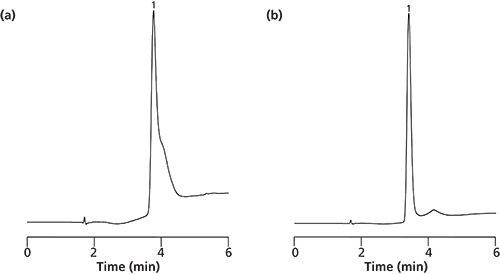
Figure 4: Separation of Gammanorm IgG on silica monoliths wide pore (30 nm) with CN-propyl surface modification. Temperature: (a) ambient and (b) 60 °C. See Figure 3 for chromatographic conditions.
Conclusions
Silica monoliths with bimodal pore structure (macro- and mesopores) are ideally suited for the chromatographic separation of biomolecules including peptides, proteins, and antibodies. Low-molecular-weight peptides can be separated on conventional silica monoliths (C18 derivatized) with 1.1-µm macropore size and 15-nm mesopore size. Antibodies and biomolecules of approximately 145 kDa molecular weight are ideally separated on silica monoliths (C4 derivatized) with 2-µm macropore- and 30-nm mesopore size. In addition, the application of temperature (up to 60 °C) further improves the analysis of antibodies, leading to optimum peak shapes needed for quantitation.
References
1. G. Walsh, in Biopharmaceuticals: An Industrial Perspective, G. Walsh and B. Murphy, Eds., (B, Kluwer Academic Publishers, Dordrecht, The Netherlands, 1999) pp. 1–34.
2. K.K. Unger, Ed., Porous Silica (Elsevier, Amsterdam, 1979).
3. K.K. Unger, N. Tanaka, and E. Machtejevas, Eds., Monolithic Silicas in Separation Science (Wiley-VCH Verlag GMBH, Weinheim, Germany, 2011).
4. G. Stegeman, R. Oostervink, J.C. Kraak, H. Poppe, and K.K. Unger, J. Chromatogr.506, 547–561 (1990).
5. N. Nimura, H. Itoh, T. Kinoshita, N. Nagae, and M. Nomura, J. Chromatogr.585(2), 207–211 (1991).
6. M. Hansen, K.K. Unger, C.T. Mant, and R.S. Hodges, TrAC 15(2), 102–110 (1996).
7. U.D. Neue, HPLC Columns (Wiley-VCH, US, 1997).
8. F. Gritti, A. Cavazzini, N. Marchetti, and G. Guiochon, J. Chromatogr.1157(1–2), 289–303 (2007).
9. J.J. Kirkland, F.A. Truszkowski, C.H. Dilks, and G.S. Engel, J. Chromatogr.890(1), 3–13 (2000).
10. B.M. Wagner, S.A. Schuster, B.E. Boyes, and J.J. Kirkland, J. Chromatogr. 1264, 22–30 (2012).
11. J.J. DeStefano, T.J. Langlois, and J.J. Kirkland, J. Chromatogr. Sci. 46(3), 254–260 (2008).
12. H. Minakuchi, K. Nakanishi, N. Soga, N. Ishizuka, and N. Tanaka, Anal. Chem.68, 3498 (1996).
13. K. Cabrera, G. Wieland, D. Lubda, K. Nakanishi, N. Soga, H. Minakuchi, and K.K. Unger, TrAC 17(1), 50–53 (1998).
14. K. Cabrera, J. Sep. Sci. 27, 843–852 (2004).
15. G. Guiochon, J. Chromatogr. A1168, 101–168 (2007).
16. S. Altmaier and K. Cabrera, J. Sep. Sci31, 2551–2559 (2008).
17. K. Cabrera, LCGC North America S4, (2012)
18. S. Fekete, J.L. Veuthey, and D. Guillarme, J. Pharm. Biomed. Anal.69, 9–27 (2012).
19. D. Guillarme, J. Ruta, S. Rudaz, and J.-L. Veuthey, Anal. Bioanal. Chem. 397(3), 1069–1089 (2010).
20. K. Vuignier, S. Fekete, P.-A. Carrupt, J.-L. Veuthey, and D. Guillarme, J. Sep. Sci.36, 2231–2243 (2013).
Karin Cabrera is R&D project manager for new silica monoliths and Chromolith HPLC columns at Merck Millipore, Advanced Analytics.
Tom Kupfer, Gisela Jung, Peter Knoell, and Benjamin Peters are coworkers in the R&D team and are responsible for the synthesis and chromatographic evaluation of silica monoliths.
E-mail: karin.cabrera@merckgroup.com
Website: www.merckmillipore.com/chromatography
This article is from The Column. The full issue can be found here>>

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.













