Application of Pyrolysis–Gas Chromatography–Mass Spectrometry for the Identification of Polymeric Materials
LCGC North America
This pyrolysis–GC–MS method enables direct analysis of solid or liquid polymers without sample pretreatment, as illustrated here for various materials, including a dental filling material and a car wrapping foil.
The analytical pyrolysis technique hyphenated to gas chromatography–mass spectrometry (GC–MS) has extended the range of possible tools for the characterization of synthetic polymers and copolymers. Pyrolysis involves thermal fragmentation of the analytical sample at temperatures of 500–1400 °C. In the presence of an inert gas, reproducible decomposition products characteristic for the original polymer or copolymer sample are formed. The pyrolysis products are chromatographically separated using a fused-silica capillary column and are subsequently identified by interpretation of the obtained mass spectra or by using mass spectra libraries. The analytical technique eliminates the need for pretreatment by performing analyses directly on the solid or liquid polymer sample. In this article, application examples of analytical pyrolysis hyphenated to GC–MS for the identification of different polymeric materials in the plastic and automotive industry, dentistry, and occupational safety are demonstrated. For the first time, results of identification of commercial light-curing dental filling material and a car wrapping foil by pyrolysis–GC–MS are presented.
Structural analysis and the study of degradation properties are important to understand and improve performance characteristics of synthetic polymers and copolymers in many industrial applications. Traditional analytical techniques used for characterization of polymers and copolymers such as thermal analysis and Fourier transform infrared (FT–IR) spectroscopy have limitations or are not sufficiently sensitive (1). Pyrolysis techniques hyphenated to gas chromatography–mass spectrometry (GC–MS) have extended the range of possible tools for the characterization of synthetic polymers and copolymers. Under controlled conditions, at elevated temperatures (500–1400 °C) in the presence of an inert gas, reproducible decomposition products characteristic for the original polymer or copolymer sample are formed. The pyrolysis products are chromatographically separated using a fused-silica capillary column and subsequently identified by interpretation of the obtained mass spectra or by using mass spectra libraries (such as the National Institute of Standards and Technology [NIST] or Wiley). Pyrolysis methods eliminate the need for pretreatment by performing analyses directly on the solid polymer or copolymer sample (1). (Please note that this article was presented at the XVII European Conference on Analytical Chemistry, which was held in Warsaw, Poland, on August 25–29, 2013).
Most of the thermal degradation results from free radical reactions initiated by bond breaking and depends on the relative strengths of the bonds that hold the molecules together. A large molecule will break apart and rearrange in a characteristic way (2–4). If the energy transfer to the sample is controlled by temperature, heating rate, and time, the fragmentation pattern is reproducible and characteristic for the original polymer or copolymer. Another sample of the same composition, heated at the same rate to the same temperature for the same period of time, will produce the same decomposition products. Therefore, the essential requirements of the apparatus in analytical pyrolysis are reproducibility of the final pyrolysis temperature, rapid temperature rise, and accurate temperature control. Depending on the heating mechanism, pyrolysis systems have been classified into two groups: the continuous-mode pyrolyzer (furnace pyrolyzer) and pulse-mode pyrolyzer (flash pyrolyzer, such as the heated filament, Curie-point, and laser pyrolyzer). The pyrolysis unit is directly connected to the injector port of a gas chromatograph. A flow of an inert carrier gas, such as helium, flushes the pyrolyzates into the fused-silica capillary column. Figure 1 shows the schematic view of the furnace pyrolyzer used in our investigation. The detection technique of the separated compounds is typically MS, but other GC detectors have also been used depending on the intentions of the analysis (1,4).

Figure 1: Schematic view of the furnace pyrolyzer used in this study.
The applications of analytical pyrolysis–GC–MS range from research and development of new materials, quality control, characterization and competitor product evaluation, medicine, biology and biotechnology, geology, airspace, and environmental analysis to forensic purposes or conservation and restoration of cultural heritage. These applications cover analysis and identification of polymers, copolymers, and additives in components of automobiles, tires, packaging materials, textile fibers, coatings, half-finished products for electronics, paints or varnishes, lacquers, leather, paper or wood products, food, pharmaceuticals, surfactants, and fragrances.
Our earlier publications (1,5–12) presented the analysis and identification of degradation products of commercially available synthetic polymers and copolymers by using analytical pyrolysis hyphenated to gas chromatography with flame ionization detection (GC–FID) and GC–MS. In this work, new examples of applications of this analytical technique for the identification of different polymeric materials are demonstrated.
Experimental
Samples
Plastic particles from industrial filter fins, a car wrapping foil, unknown fibers, and commercial light-curing dental filling material were used in the investigation.
Instrumentation and Analytical Conditions
Approximately 100–200 µg of solid sample was cut out with a scalpel and inserted without any further preparation into the bore of the pyrolysis solids-injector and then placed with the plunger on the quartz wool of the quartz tube of the furnace pyrolyzer Pyrojector II (SGE Analytical Science). Three spots on each sample were analyzed in duplicate. The pyrolyzer was operated at a constant temperature of 550, 600, 700, or 900 °C. The pressure of helium carrier gas at the inlet to the furnace was 95 kPa.
Pyrolysis–GC–MS measurements were made using two apparatus. In the first apparatus (1), the pyrolyzer was connected to a Trace 2000 gas chromatograph (ThermoQuest, CE Instruments) with a quadrupole mass spectrometer Voyager (ThermoQuest, Finnigan, MassLab Group) operated in electron ionization (EI) mode. A 60 m × 0.25 mm, 0.25-µm Elite-5ms fused-silica GC capillary column (PerkinElmer Instruments) was used. The GC conditions were as follows: programmed column temperature: 60 °C for 1 min, then 60–100 °C at 2.5 °C/min, 100–280 °C at 10 °C/min (20-min hold at 280 °C). The temperature of the split–splitless injector was 250 °C and the split flow was 50 cm3/min. Helium, grade 5.0 (Westfalen AG), was used as a carrier gas. The helium programmed pressure was 70 kPa for 1 min, then 70–110 kPa at 1 kPa/min (hold at 110 kPa to the end of analysis) was used. The transfer line temperature was 280 °C. The MS EI ion source temperature was kept at 250 °C. The ionization occurred with a kinetic energy of the impacting electrons of 70 eV. The current emission of the rhenium filament was 150 µA. The MS detector voltage was 350 V. Mass spectra and reconstructed chromatograms (total ion current [TIC]) were obtained by automatic scanning in the mass range m/z 35–450 u. Pyrolysis–GC–MS data were processed with the Xcalibur software (ThermoQuest) and the NIST 05 mass spectra library.
In the second apparatus (2), the pyrolyzer was connected to a 7890A gas chromatograph with a series 5975C quadrupole mass spectrometer (Agilent Technologies Inc.) operated in EI mode. A 59 m × 0.25 mm, 0.25-µm df DB-5ms fused-silica GC capillary column (J&W Scientific) was used. Helium, grade 5.0 (Westfalen AG), was used as a carrier gas. The GC conditions were as follows: programmed column temperature: 60 °C for 1 min, then 60–280 °C at 7 °C/min (hold at 280 °C to the end of analysis); programmed helium pressure: 122.2 kPa for 1 min, then 122.2–212.9 kPa at 7 kPa/min (hold at 212.9 kPa to the end of analysis). Second set of GC conditions: programmed column temperature: 75 °C for 1 min, then 75–280 °C at 7 °C/min (hold at 280 °C to the end of analysis); programmed helium pressure: 122.2 kPa for 1 min, then 122.2–212.9 kPa at 7 kPa/min (hold at 212.9 kPa to the end of analysis).
The temperature of the split–splitless injector was 250 °C and the split ratio was 50:1. The transfer line temperature was 280 °C. The MS EI ion source temperature was kept at 230 °C. The ionization occurred with a kinetic energy of the impacting electrons of 70 eV. The quadrupole temperature was 150 °C. Mass spectra and reconstructed chromatograms (total ion current) were obtained by automatic scanning in the mass range m/z 35–750 u. Pyrolysis–GC–MS data were processed with the ChemStation software (Agilent Technologies) and the NIST 05 mass spectra library.

Figure 2: PyrolysisâGCâMS chromatogram of plastic particles from industrial filter fins at 700 °C obtained with apparatus 1. Fused-silica GC capillary column: 60 m à 0.25 mm, 0.25-µm df Elite-5ms. GC conditions: programmed column temperature: 60 °C for 1 min, then 60â100 °C at 2.5 °C/min and then 100â280 °C at 10 °C/min (20 min hold at 280 °C); splitâsplitless injector temperature: 250 °C; split flow: 50 cm3/min; helium programmed pressure: 70 kPa for 1 min, then 70â110 kPa at 1 kPa/min (hold at 110 kPa to the end of analysis). For peak identification, see Table I.
Results and Discussion
Pyrolysis–GC–MS of Plastic Particles from Industrial Filter Fins
A sample of plastic particles from industrial filter fins was pyrolyzed at 700 °C to identify its composition. Figure 2 shows the obtained pyrolysis–GC–MS chromatogram of the sample. Based on the decomposition products summarized in Table I, the plastic particles were identified as a mixture of poly(hexamethylene adipamide) (nylon 6-6) and polypropylene glycol with a small amount of styrene–butadiene-rubber (SBR). The peaks of propylene and acetone indicate the presence of polypropylene glycol. The main decomposition product of nylon 6-6 is cyclopentanone (retention time [tR] = 10.51 min). Other peaks in Figure 2, like butene/1,3-butadiene (tR = 5.58 min), benzene (tR = 7.43 min), toluene (tR = 9.72 min), and styrene (tR = 14.39 min) are typical pyrolysis products of SBR (1,2,5,6,18). The small peak of 4-isopropylphenol (tR = 28.15 min) may be a clue to the presence of polycarbonate or bisphenol A epoxy resin (5,6). All of the pyrolysis products and the materials identified from pyrolysis products in filter fins are summarized in Table I.
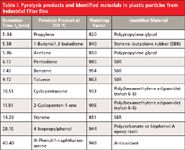
Table I: Pyrolysis products and identified materials in plastic particles from industrial filter fins
Pyrolysis–GC–MS of a Car Wrapping Foil
The next object of identification was a car wrapping foil pyrolyzed at 600 °C. Figure 3 shows the obtained pyrolysis–GC–MS chromatogram of the car wrapping foil. Based on the decomposition products summarized in Table II, the plastic material was identified as a mixture of flexible poly(vinyl chloride) (PVC) with bis(2-ethylhexyl) phthalate (BEHP) plasticizer and poly(hexamethylene adipamide) (nylon 6-6). The chromatogram in Figure 3 shows the typical pyrolysis products of PVC, like hydrogen chloride (tR = 5.49 min), benzene (tR = 7.48 min), and naphthalene (tR = 25.93 min). This is the result of the formation of double bonds by the elimination of hydrogen chloride from the poly(vinyl chloride) macromolecules, followed by the breaking of the carbon chain with or without cyclization reaction (2). The detected cyclopentanone (tR = 10.46 min) is generally known as characteristic pyrolysis product of nylon 6-6 (2,3,6). Methyl methacrylate (tR = 8.20 min) identified in pyrolyzate is formed from poly(methyl methacrylate) (6) and most likely comes from the adhesive film. Thus, the identified 3,3-diphenylacrylonitrile (tR = 36.69 min) may be from the adhesive layer of the foil.
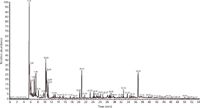
Figure 3: PyrolysisâGCâMS chromatogram of a car wrapping material at 600 °C obtained with apparatus 1. Fused-silica GC capillary column 60 m à 0.25 mm, 0.25-µm df Elite-5ms. GC conditions: programmed column temperature: 60 °C for 1 min, then 60â100 °C at 2.5 °C/min and then 100â280 °C at 10 °C/min (20 min hold at 280 °C); splitâsplitless injector temperature: 250 °C; split flow: 50 cm3/min; helium programmed pressure: 70 kPa for 1 min, then 70â110 kPa at 1 kPa/min (hold at 110 kPa to the end of analysis). For peak identification, see Table II.
The thermal decomposition of the plasticizer bis(2-ethylhexyl) phthalate identified in car wrapping foil leads to the formation at 600 °C of 2-ethyl-1-hexene (tR = 10.29 min), 2-ethylhexanal (tR = 17.33 min), 2-ethyl-1-hexanol (tR = 20.57 min), and phthalic anhydride (tR= 28.63 min) (1,7). In the car wrapping material, the rest of the tetrahydrofuran solvent (tR = 6.99 min) was also detected. Table II shows the identified ingredients of the pyrolyzed car wrapping foil.

Table II: Pyrolysis products and identified materials in car wrapping foil
Identification of Unknown Plastic Fibers
A sample of unknown plastic fibers was pyrolyzed at 700 °C and 900 °C, respectively, to identify its composition. Figure 4 shows the pyrolysis–GC–MS chromatogram of the sample pyrolyzed at 900 °C. Based on the decomposition products summarized in Table III, the fibers were identified as polyaramid [poly(p-phenylene terephthalamide)] (Figure 5). The main identified degradation products of polyaramid at 900 °C are benzene (tR = 7.56), aniline (tR = 11.61 min), and benzonitrile (tR = 11.81 min). Currently, polyaramid fibers have only been characterized in a few publications using thermal analysis (thermogravimetry, derivative thermogravimetry, and differential thermal analysis), infrared spectroscopy techniques (13–15), and pyrolysis–GC–MS (2,16–19).

Figure 4: PyrolysisâGCâMS chromatogram of polyaramid fibers at 900 °C obtained with apparatus 2. Fused-silica GC capillary column: 59 m à 0.25 mm, 0.25-µm df DB-5ms. GC conditions: programmed column temperature: 75 °C for 1 min, then 75â280 °C at 7 °C/min (hold to the end of analysis); programmed pressure of helium carrier gas: 122.2 kPa for 1 min, then 122.2â212.9 kPa at 7 kPa/min (hold at 212.9 kPa to the end of analysis). For peak identification, see Table III.
Polyaramid fibers are a class of heat-resistant, strong synthetic fibers. They are used in aerospace and military applications for ballistic-rated body armor, fabric, ballistic composites, and fire fighters protective clothing as well as in bicycle tires and as an asbestos substitute.
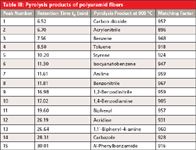
Table III: Pyrolysis products of polyaramid fibers
Identification of Commercial Light-Curing Dental Filling Material
A number of dental filling materials are presently available for tooth restorations. The four main groups of these materials, which dentists have used for about 35 years, are the conventional glass-ionomer cements, resin-based composites, resin-modified glass-ionomer cements, and polyacid-modified resinous composites (20). Light-curing glass-ionomer cements contain polyacrylic acid, chemically or photo-curing monomers (multifunctional methacrylates, like triethylene glycol dimethacrylate or 2-hydroxyethyl methacrylate), an ion-leaching glass, and additives (initiators, inhibitors, stabilizers, and others) (20). Resin-modified glass-ionomer cements are now widely used in dentistry as direct filling materials, liners, bases, luting cements, and fissure sealants (21). These materials mainly consist of polymer matrix and glass-ionomer parts. The polymer matrix is based on a monomer system and different multifunctional methacrylates with additives (21). Methacrylic monomers, such as bisphenol A glycidyl methacrylate (Bis-GMA), urethane dimethacrylate (UDMA), triethylene glycol dimethacrylate (TEGDMA), and 2-hydroxyethyl methacrylate (HEMA), are the main components of resin-based dental filling materials. The presence of additives such as initiators, activators, inhibitors, and plasticizers in uncured dental material mixture is necessary (21).
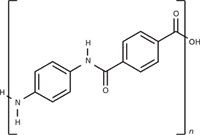
Figure 5: Chemical structure of poly(p-phenylene terephthalamide) (polyaramid).
Figure 6 shows the total ion current pyrolysis–GC–MS chromatogram of commercial light-curing dental filling material pyrolyzed at 550 °C. The pyrolysis products identified by using mass spectra library NIST 05 are summarized in Table IV. The carbon dioxide (tR = 6.85 min) identified in pyrolyzate is formed from polyacrylic acid (2,18). The identified substances 2-hydroxyethyl methacrylate (HEMA) (tR = 13.65 min), ethylene glycol dimethacrylate (EGDMA) (tR = 19.48 min), and triethylene glycol dimethacrylate (TEDMA) (tR = 28.72 min) are known as standard composites of dental filling materials (1). Other compounds in Table IV, such as bisphenol A (tR = 33.10 min) or bisphenol A diglycidyl ether (tR = 42.42 min), are probably formed by thermal degradation of bisphenol A diglycidyl mono- or dimethacrylates. The presence of the additives, such as the antioxidant 2,6-bis(1,1-dimethylethyl)-4-methylphenol (BHT) (tR = 23.17 min) or the UV-absorber drometrizol (tR = 31.95 min) was also confirmed. The triphenylantimony (tR = 34.55 min) identified in pyrolyzate is used as catalyst in the UV-induced polymerization (1).

Figure 6: PyrolysisâGCâMS chromatogram of commercial light-curing dental filling material at 550 °C obtained with apparatus 2. Fused-silica GC capillary column: 59 m à 0.25 mm, 0.25-µm df DB-5ms. GC conditions: programmed column temperature: 60 °C for 1 min, then 60â280 °C at 7 °C/min (hold at 280 °C to the end of analysis); programmed helium pressure: 122.2 kPa for 1 min, then 122.2â212.9 kPa at 7 kPa/min (hold at 212.9 kPa to the end of analysis). For peak identification, see Table IV.
Conclusion
Analytical pyrolysis–GC–MS has been proven as a valuable technique for the analysis and identification of organic polymeric materials in the plastic and rubber industry. For the first time pyrolysis–GC–MS was used for the identification of commercial light-curing dental filling material and for the identification of a car wrapping foil. This technique allows the direct analysis of very small sample amounts (5–200 µg) without the need for time-consuming sample preparation.

Table IV: Pyrolysis products of commercial light-curing dental filling material
References
(1) P. Kusch, in Advanced Gas Chromatography – Progress in Agricultural, Biomedical and Industrial Applications, M.A. Mohd, Ed. (InTech, Rijeka, Croatia, 2012), pp. 343–362.
(2) S.C. Moldoveanu, Analytical Pyrolysis of Synthetic Organic Polymers (Elsevier, Amsterdam, The Netherlands, 2005).
(3) T.P. Wampler, Applied Pyrolysis Handbook, 2nd Ed. (CRC Press, Boca Raton, Florida, 2007).
(4) K.L. Sobeih, M. Baron, and J. Gonzales-Rodrigues, J. Chromatogr. A 1186, 51–66 (2008).
(5) P. Kusch, Chem. Anal. (Warsaw) 41, 241–252 (1996).
(6) P. Kusch, G. Knupp, and A. Morrisson, in Horizons in Polymer Research, R.K. Bregg, Ed. (Nova Science Publishers, New York, New York, 2005), pp. 141–191.
(7) P. Kusch, LCGC North Am. 31(3), 248–254 (2013).
(8) P. Kusch, V. Obst, D. Schroeder-Obst, W. Fink, G. Knupp, and J. Steinhaus, Eng. Fail. Anal. 35, 114–124 (2013).
(9) P. Kusch and G. Knupp, Nachr. Chem. 57, 682–685 (2009).
(10) P. Kusch, V. Obst, D. Schroeder-Obst, G. Knupp, and W. Fink, LCGC AdS, July/August, 5–11 (2008).
(11) P. Kusch and G. Knupp, LCGC AdS, June, 28–34 (2007).
(12) P. Kusch, W. Fink, D. Schroeder-Obst, and V. Obst, Aluminium (Isernhagen, Germany) 84(4), 76–79 (2008).
(13) S. Villar-Rodil, A. Martínez-Alonso, and J.M.D. Tascón, J. Anal. Appl. Pyrol. 58–59, 105–115 (2001).
(14) F. Suárez-García, A. Martínez-Alonso, and J.M.D. Tascón, Carbon 42, 1419–1426 (2004).
(15) S. Villar-Rodil, A. Martínez-Alonso, and J.M.D. Tascón, J. Therm. Anal. Calorim. 79, 529–532 (2005).
(16) J.R. Brown and A.J. Power, Polym. Degrad. Stab. 4(5), 379–392 (1982).
(17) H.-R. Schulten, B. Plage, H. Ohtani, and S. Tsuge, Angew. Makromol. Chem. 155, 1–20 (1987).
(18) S. Tsuge, H. Ohtani, and C. Watanabe, Pyrolysis-GC/MS Data Book of SyntheticPolymers (Elsevier, Amsterdam, The Netherlands, 2011).
(19) Zs. Czégény and M. Blazsó, J. Anal. Appl. Pyrol. 58–59, 95–104 (2001).
(20) R. Rogalewicz, A. Voelkel, and I. Kownacki, J. Environ. Monit. 8, 377–383 (2006).
(21) R. Rogalewicz, K. Batko, and A. Voelkel, J. Environ. Monit. 8, 750–758 (2006).
Peter Kusch, Gerd Knupp, Wolfgang Fink, Dorothee Schroeder-Obst, and Johannes Steinhaus are with Hochschule Bonn-Rhein-Sieg, University of Applied Sciences in the Department of Applied Natural Sciences, in Rheinbach, Germany. Volker Obst is with Dr. Obst Technische Werkstoffe GmbH, in Rheinbach, Germany. Direct correspondence to: Peter.Kusch@h-brs.de

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











