Analysis of Polyether Antibiotics in Animal Feeds by HPLC with Post-Column Derivatization
Polyether antibiotics are commonly used for preventing coccidiosis and other infections in poultry and for improving feed efficiency for beef cattle and swine.
Polyether antibiotics are commonly used for preventing coccidiosis and other infections in poultry and for improving feed efficiency for beef cattle and swine. The use of polyether antibiotics is strictly regulated, with only specific ionophores approved for use in feeds intended for different animals.
Analysis of polyether antibiotics by HPLC with post-column derivatization and UV–vis detection has been proven to successfully identify and quantify monensin, narasin, and salinomycin in medicated feeds, supplements, and premixes as well as to determine trace contamination levels in non-medicated feeds (1,2).
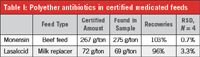
Table I: Polyether antibiotics in certified medicated feeds
Post-column derivatization of polyether antibiotics is done using highly acidic vanillin or DMAB reagents. Pinnacle PCX derivatization system (Pickering Laboratories, Inc.) has an inert flow path and automated system wash capabilities that make it uniquely suitable for handling corrosive reagents. The two-pump system is recommended to extend reagent stability, but the single-pump system for this application is also available.
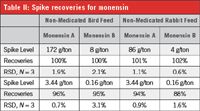
Table II: Spike recoveries for monensin
Adding a fluorescence detector to the instrumentation allows for using the same extraction procedure and HPLC conditions to also determine lasalocid which doesn't require post-column derivatization.
Method
Sample Preparation
To 25 g of finely ground feed sample, add 100 mL of extraction solution (90% methanol–10% water). Shake for 1 h at high speed using mechanical shaker. Let the solids settle and filter an aliquot of the extract for injection. Dilute with extraction solution if needed to fit the calibration curve. Use 2.5 g portion when testing premixes.

Figure 1: Standard mixture of monensin, salinomycin, and narasin.
Analytical conditions
Analytical Column: Polyether Column, C18, 4.6 × 250 mm, Catalog No. 2381750
Temperature: 40 °C
Flow rate: 0.7 mL/min
Mobile Phase: 90% methanol, 10% of 5% acetic acid solution in water, isocratic
Injection volume: 20 µL

Figure 2: Certified medicated beef feed sample containing 267 g/ton of monensin.
Post-Column Conditions
Post-column System: Pinnacle PCX
Reactor Volume: 1.4 mL
Reactor Temperature: 90 °C
Reagent 1: Concentrated sulfuric acid/methanol (4:96 v/v)
Reagent 2: 60 g of vanillin in 950 mL of methanol
Reagents Flow Rate: 0.3 mL/min
Detection: UV-vis 520 nm (for Lasalocid – FLD, Ex. 322 nm, Em. 370 nm)
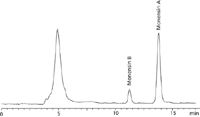
Figure 3: Non-medicated bird feed sample spiked with monensin A (3.44 µg/g) and monensin B (0.16 µg/g).
Calibration
Monensin A: 0.1–50 ppm, R2 = 0.999
Monensin B: 0.0035–0.7 ppm, R2 = 0.999
Lasalocid acid: 0.25–50 ppm, R2 = 0.999
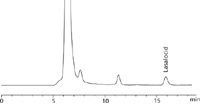
Figure 4: Certified medicated milk replacer containing 72 g/ton of lasalocid.
Conclusion
Analysis of polyether antibiotics by HPLC with post-column derivatization is a robust and sensitive method that utilizes standard equipment and could easily be adopted by testing laboratories. It allows for testing of different ionophores at wide range of concentrations, including at trace levels. Using Pinnacle PCX post-column derivatization system, factory configured for the analysis, guarantees stable and reproducible results.
References
(1) H. Campbell and G. Nayeri, J. AOAC Int. 89, 1229–1242 (2006).
(2) AOAC Official Method 997.04. Monensin in Premix and Animal Feeds.

Pickering Laboratories, Inc.
1280 Space Park Way, Mountain View, CA 94043
tel. (800) 654-3330, (650) 694-6700
Website: www.pickeringlabs.com
















