Analysis of PFAS in Locally Acquired Food Containers
In this study, we have combined both an untargeted approach using ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UHPLC–QTOF) and a targeted approach using an UHPLC-tandem mass spectrometry (MS/ MS), to detect the presence of PFAS in samples of food containers acquired randomly from local fast food establishments from the region. This technical article reports on a survey where untargeted UHPLC–QTOF analyses was first performed and then followed, when possible, by targeted UHPLC–MS/MS, to detect which PFAS may be present in locally acquired food containers. Of the 14 samples of food containers tested, 10 showed possible PFAS contamination using untargeted analysis. The specific PFAS detected ranged from PFAS common in the literature, such as PFBA and PFHxA, to certain PFAS rarely discussed in the literature, such as PSPDA and N-EtPBSA. Targeted analysis showed five samples contaminated by three different PFAS with concentrations similar to those found elsewhere in the literature. These results highlight the persistent prevalence of PFAS in food containers despite new regulation efforts, and suggest manufacturers may be incorporating other PFAS in response to evolving regulations.
Per- and poly-fluorinated alkyl substances (PFAS) are human manufactured chemicals that are at least partially soluble in water and oil, and resistant to heat. These properties have made them commonplace in many everyday items such as clothing, furniture, food packaging, and kitchenware (1‒3). As such, humans are often exposed to PFAS contamination through ingestion of food, water, and inhalation of dust. Despite their common uses, there is a growing body of reports linking PFAS to various health complications, including bioaccumulation, cancer, immune system suppression, impaired growth, altered thyroid function, liver damage, and reproductive complications (4).
Eco-friendly tableware - kraft paper food packaging on light green background. Street food paper packaging, recyclable paperware, zero waste packaging concept. Flat lay | Image Credit: © Iryna Mylinska - stock.adobe.com

Due to these health risks and low biodegradability rates, in 2006 the U.S. Environmental Protection Agency (EPA) worked with leading chemical manufacturers to voluntarily end the production by 2015 of two types of long chain PFAS: perfluoroalkylcarboxylic acid (PFOA) and perfluorosulfonic acid (PFOS), as pictured in Figure 1 (5). Around that time, the European Union initiated a similar program to phase out long chain PFAS (6). However, both PFOA and PFOS can still be imported from international manufacturers, and due to their long term ("legacy") characteristics can still be found in some consumer goods. Manufacturers have responded to the phase out of PFOA and PFOS by replacing them with shorter fluorinated substances, at times modified by different functional groups but with similar, effective industrial uses. State and local regulations have changed as new shorter chain PFAS have been allowed to be incorporated in products such as consumer goods. These short chain PFAS and their derivatives have been found to have shorter biological and environmental half-lives, and have an increased level of solubility, but have been found to cause many of the same health effects (1).
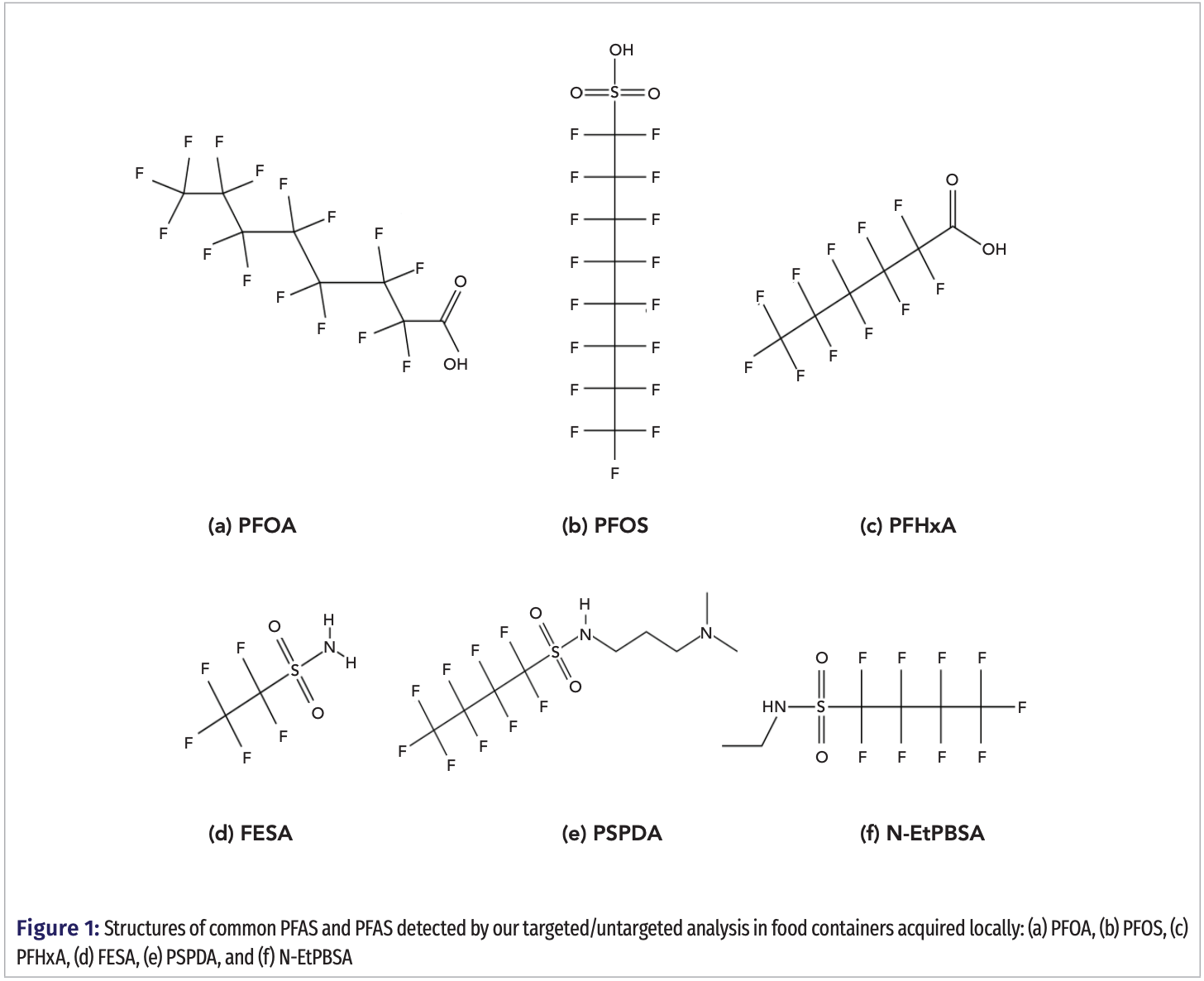
Glenn and associates recently published a comprehensive review of PFAS on all aspects of their historical development, commercial manufacturer, bio-accumulative effects, uses in food contact packaging and paperboard, including the need to find viable replacements. From the various studies cited, the sources of PFAS human chronic exposure come from food, drinking water, and dust, with the most important exposure coming from the migration of PFAS from food packaging (1). Ongoing nationwide monitoring has existed by the U.S. Food and Drug Administration’s (FDA’s) “Total Diet Program” that annually report the levels of 16 PFAS found in milk, cheese, bread, meat, fish, and other produces (7). Relevant to this present study, Schaider and associates reported in 2017 on the collection and testing of over 400 different samples of food packaging from various fast food restaurants. Screening first was done to detect the presence of total fluorine using particle-induced gamma emission (PIGE) spectroscopy, and then a select subset of twenty samples was analyzed by liquid chromatography–high resolution mass spectrometry (LC–HRMS), using both targeted and untargeted methods. In summary, fluorine was detected by PIGE in 56% of the bread and dessert wrappers, in 38% of the burger and sandwich wrappers, in 20% of paper board-bowls and plates, while there was no fluorine detected in paper cups (for hot and cold drinks) (8). In an interesting report with statistical evaluation of the 2003‒2014 U.S. National Health and Nutrition Survey, it was reported that PFAS levels found in human serum showed limited statistical correlations of foods consumed from fast food and pizza restaurants in relation to the foods cooked at home. However, it was noted that a significant correlation of higher PFAS levels were found in human serum with the consumption of microwave popcorn (9). In various other studies from the United States and countries abroad, it was reported that the most prevalent and highest levels of PFAS were detected in microwave popcorn (10,11). In recent studies, certain PFAS were reported to have lower longevity and less harmful health effects, and consequently received regulatory approval to be manufactured into specific food packaging materials (1). Attention needs to be given to the fact that trace levels of restricted PFAS can still be detected as a result of residues coming from recycled materials or unintentional laboratory contamination (12). In 2019, Li and co-authors emphasized how effectively simulant solution studies, in combination with an integrated analytical approach of LC–MS/MS and GC–MS/MS, can increase the scope in identifying which PFAS can migrate from food contact materials into foods (13). In 2022, Barhoumi and associates published an extensive review of the detection, accumulation and release of various PFAS onto microplastics and food-contact materials (14). While also on 2022, Carmero and co-authors reported an interesting review on the detections of PFAS in fast food packaging, microwave popcorn bags, and frying pans and their migration into foods (15).
In this study, we have combined both an untargeted approach using ultra-high performance liquid chromatography–quadrupole time-of-flight (UHPLC–QTOF) MS, and a targeted approach using an UHPLC–MS/MS, to detect the presence of PFAS in representative samples acquired from local fast-food establishments. Our targeted analysis focused on identifying and quantifying 14 PFAS which are included in EPA537 method (16). The untargeted analysis allowed for possible identification of PFAS utilizing the PFAS compound library of over 250 PFAS (included with the Sciex Operating System), without any need for standards, or prior knowledge of which PFAS a sample might contain. In total, 14 samples of local food containers were collected from various local fast food establishments, which included components of grinder bags, takeout containers, soup containers, hot cups, a salad box, and a potato chip bag. These samples were chosen based on the high levels of PFAS contamination previously reported (8,12).
Experimental
Materials and Reagents
LC–MS grade methanol, water and ammonium acetate were purchased from Thermo-Fisher Chemical. Individual PFAS standards and the EPA Method 537.1 standard mixture were purchased from Sigma-Aldrich and AccuStandard Inc. The internal standard solution mixture (EPA-537IS) and the surrogate standard solution mixture (EPA-537SS) were purchased from Wellington Laboratories.
Various food containers were collected randomly from local fast food establishments around the region. Care was taken to use only polyethylene (PE) or polypropylene (PP) sample storage containers, gloves and other laboratory supplies. In the sample preparations steps only PE centrifuge tubes, (PE 15-mL, Thermo-Fisher) and UHPLC vials of PE screw caps vials (Microsolv) equipped with 300 μL inserts (Thermo-Fisher) and with PE screw caps (Microsolv) were used.
Sample preparation steps were performed in a clean room as a precaution to further minimize PFAS contamination.
Sample Preparation Method
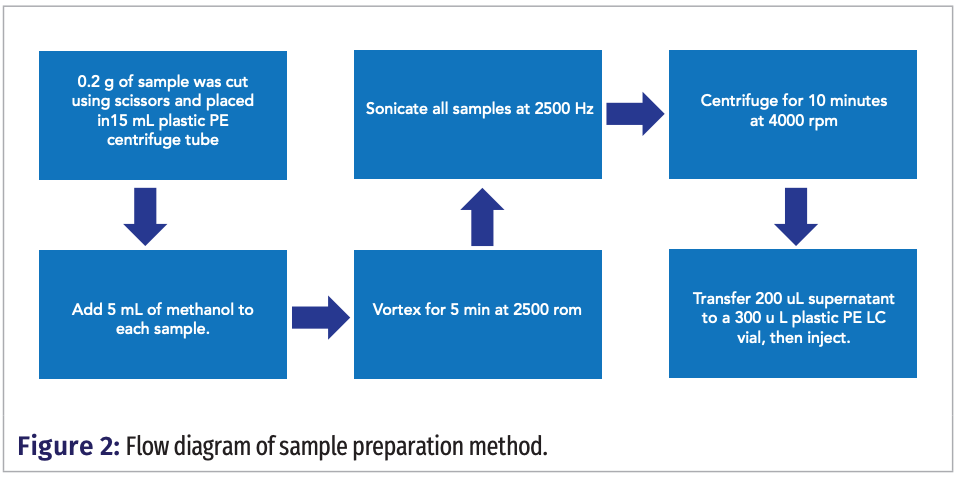
The sample preparation method involving extraction with methanol was designed to maximize the detection of all PFAS present in samples. The standard operation procedure for sample preparation, depicted in Figure 2 was performed as follows: a 0.2 g piece of each food container was cut using scissors and placed into a 15 mL plastic centrifuge tube. Then 5 mL of methanol was added to each sample. Samples were sonicated at 2500 Hz, and then vortexed for 5 min at 2500 rpm. Sonication serves to break up material fibers while vortexing facilitates the entry of PFAS into the methanol solution. Afterwards, samples were centrifuged for 10 min at 4000 rpm. Then 200 μL of supernatant was transferred to a 300 μL plastic PE LC vial, followed by MS analyses.
Instrumentation
The untargeted analysis was performed on a Sciex Exion UHPLC coupled with a Sciex X500R Quadrupole/Time of Flight (AB Sciex) fitted with a PFAS conversion kit. An Acquity UPLC BEH C18 (1.7 µm, 2.1 x 50 mm) column (Waters), heated to 50 °C with a sample injection volume of 10 µL on a 20 µL loop, was utilized for analyte separation. The mobile phase consisted of 2 mM ammonium acetate in 95:5 water:methanol (solvent A) and 2mM ammonium acetate in methanol (solvent B), was employed for gradient column elution. The total run time was 9.0 min with a constant flow rate of 0.4 mL/min. Table I shows the solvent gradient used for the targeted and untargeted analysis.
The detection and relative quantification of PFAS were performed in ESI-SWATH mode for untargeted analysis. The Sciex OS software was utilized for guided analyte signal optimization. Statistical analysis for obtaining identification and quantification results for all compounds were performed using Analytics and the PFAS compound library (which at the time contained 240 PFAS), which are included in the Sciex OS software v.2.0.1. The software categorizes compound detection probability based on compound fragmentation patterns, precursor mass error, isotope patterns, and elution time that matches compound polarity. Thus, detected compounds were characterized as “possibly detected” (pd) if their potential peak match has a probability above 0.8 as set as default in the software.
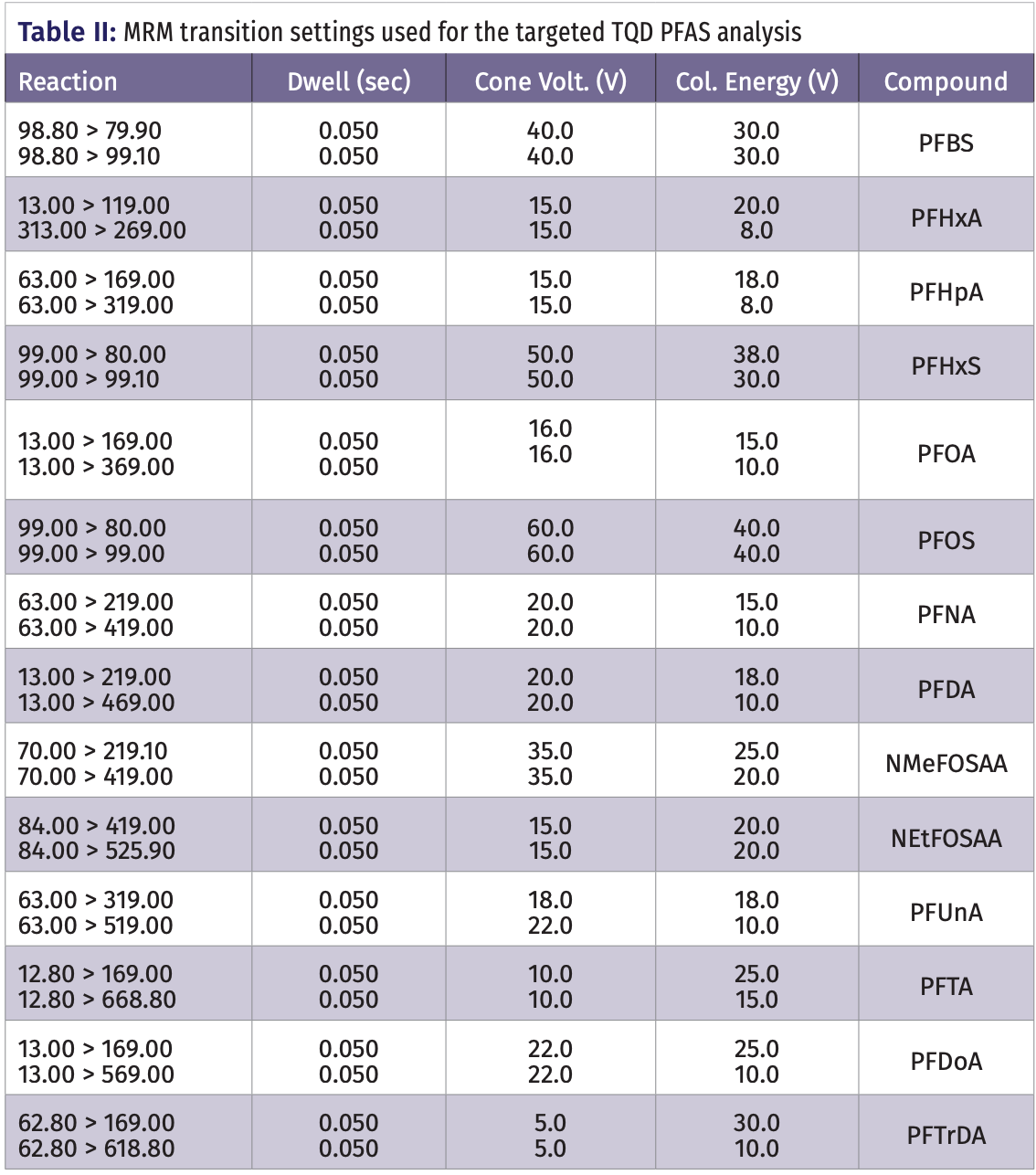
The targeted analysis was performed using a Waters Acquity UPLC coupled with an Acquity TQD triple quadrupole tandem mass spectrometer (Waters). The same column, heating conditions, inject conditions, mobile phase conditions, and runtime from the untargeted analysis were used with this instrument. The targeted detection and quantification of analytes and surrogate compounds were performed in negative ESI–MS/MS mode (MRM) using the Waters IntelliStart software for analyte signal optimization. Statistical analysis for obtaining calibration and quantification results for all compounds were performed using Waters QuanLyn, which is included in the MassLynx software v.4.2. The UPLC solvent gradient program is shown in Table I. The TQD mass spectrometric MRM transitions are shown in Table II.
Results and Discussion
Of the 14 samples of food containers tested, 10 showed possible PFAS contamination using untargeted analysis. Table III and Table IV report on which food wraps were sampled and which of the PFAS were found in each food container studied. Of interest was that both of the outer sandwich paper wraps showed the possible detection of PFEA or PSPDA, while the innermost sandwich paper wraps, which were directly exposed to the actual food, showed no detection of any PFAS. One of the sandwich bags, both hot cups and the hot soup container showed the presence of PFBA, PSPDA, or PFHxA. Most relevant was that the potato chip bag showed the presence of the shorter N-EtPBSA and FESA, but not the longer chain PFOS. The specific PFAS detected ranged from PFAS common in the literature, such as PFBA and PFHxA, to PFAS rarely discussed in the literature such as PSPDA and N-EtPBSA. The detection of little know PFAS is not unexpected, as manufacturers are continuously changing which PFAS are used in response to evolving guidelines from state and federal regulatory agencies. This fact highlights the need for untargeted analysis research that is capable of identifying new PFAS as they are implemented in the market. It is noted that the results obtained were based on the extraction of food containers with the methanol solvent extraction approach which may not reflect the PFAS that transfer to food from the containers.
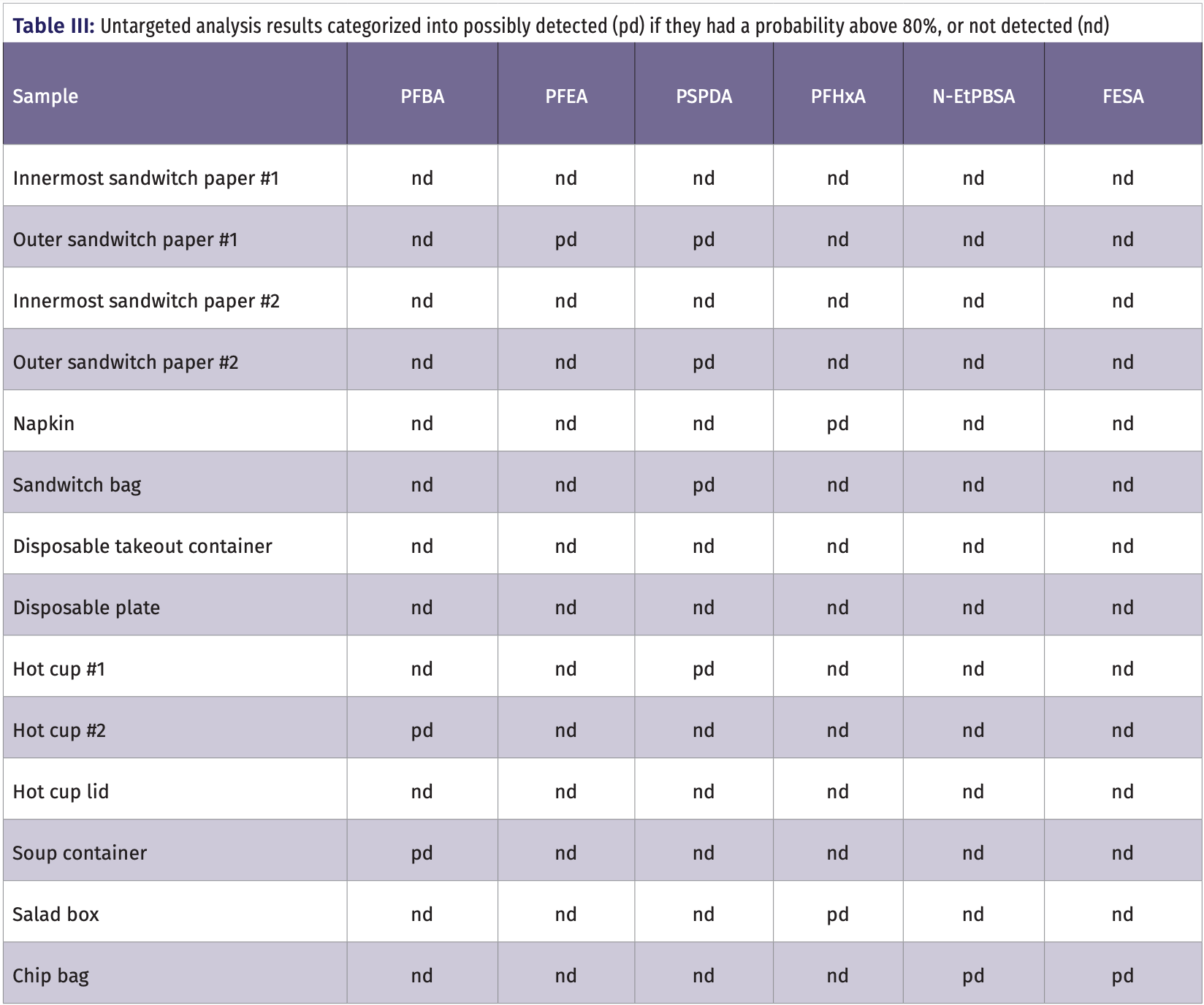
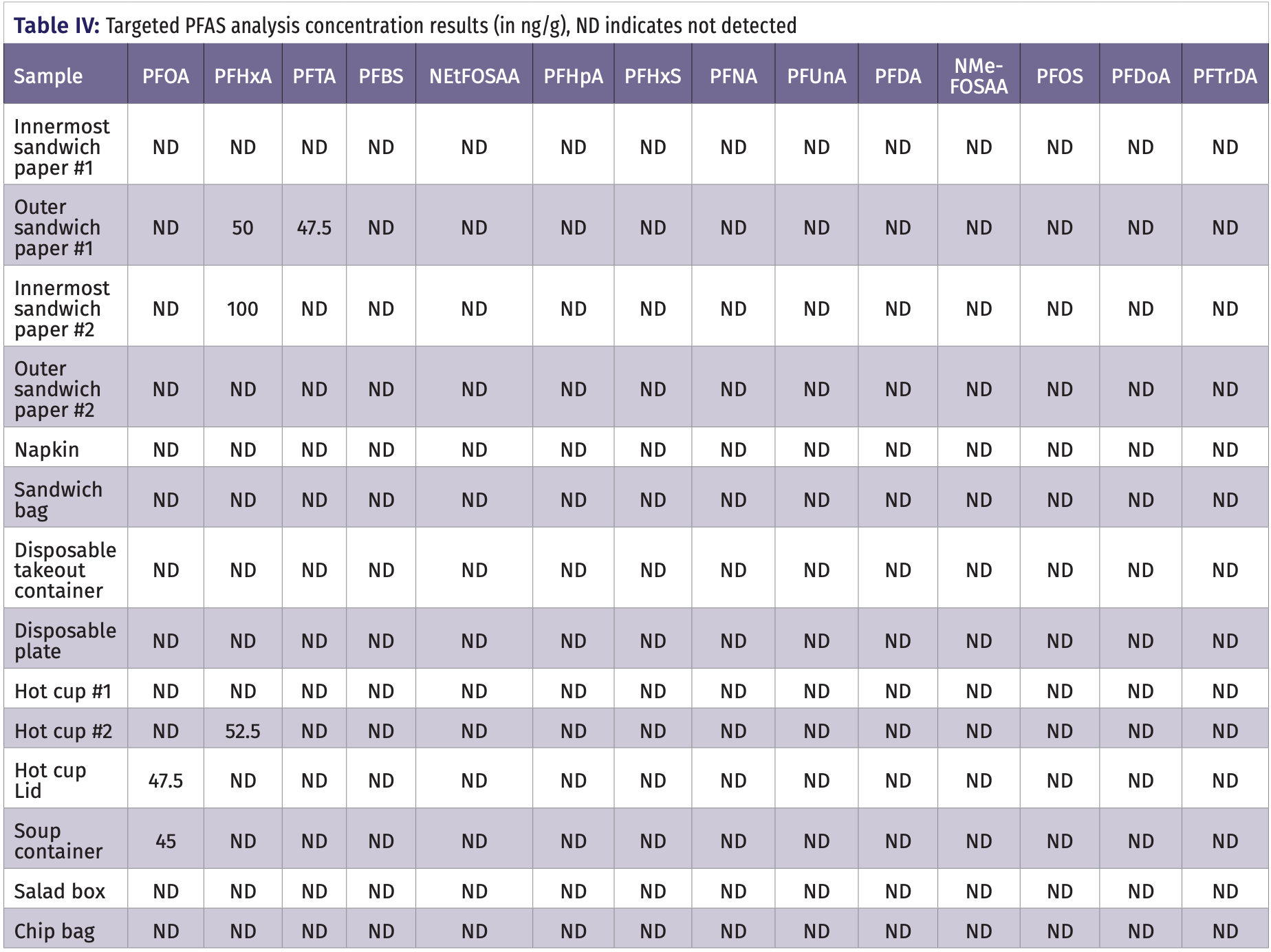
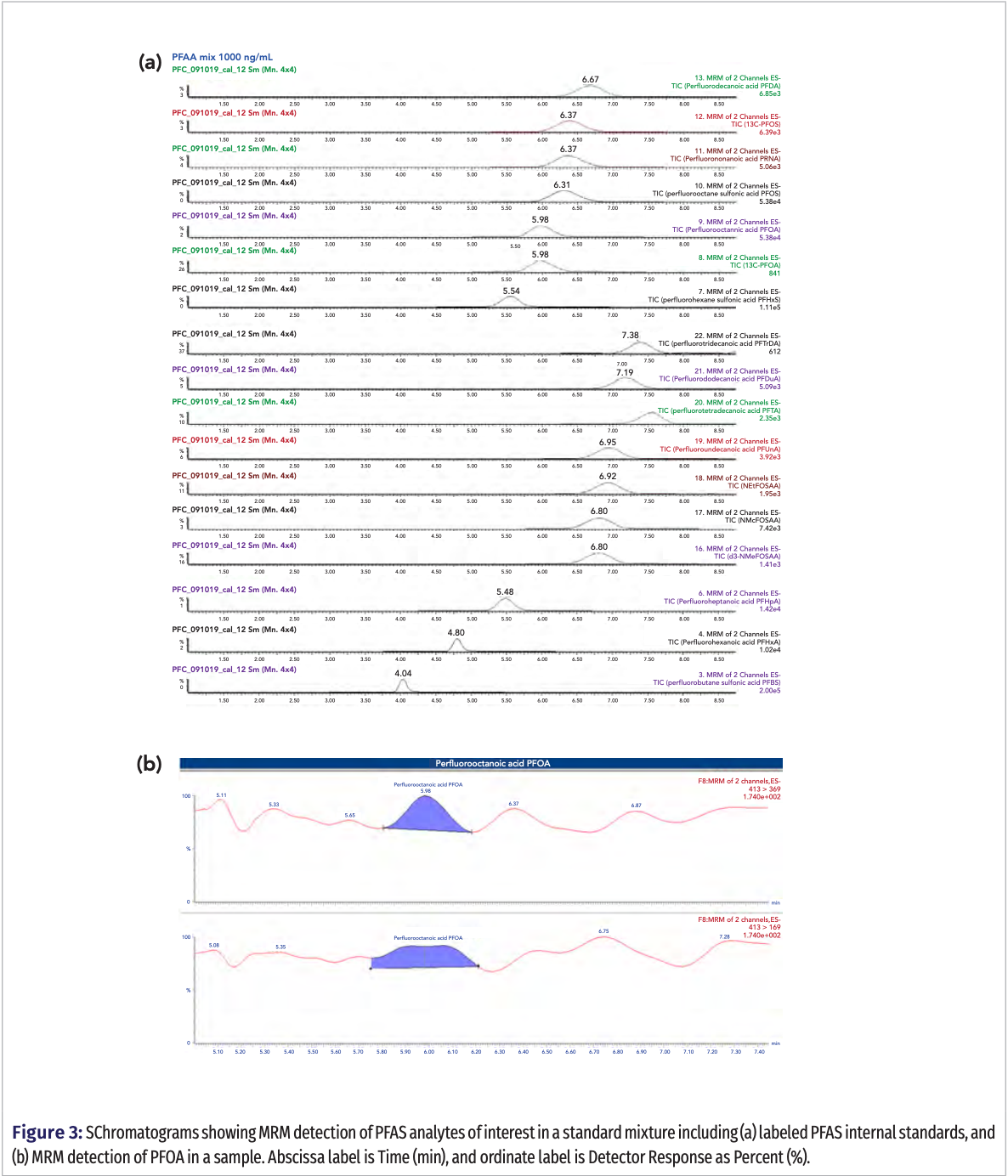
Targeted analysis showed five samples contaminated with three different PFAS with concentrations mostly around 50 ng/g, similar to those found elsewhere in the literature (1‒3,11,12). In confirmation with the untargeted analysis, the outer sandwich wrap paper was found to have a concentration of 47.7 ng/g PFTA and 50.0 ng/g for PFHxA. For hot cup 2, its lid and the soup container also showed similar concentrations with 52.5 ng/g PFHxA, 47.5 ng/g PFOA, and 45.0 ng/g respectively. Figure 3a shows the chromatograms for the targeted UHPLC–MS/MS analysis with MRM detection of a standard mixture of the 14 PFAS at the 25ng/mL concentration level and Figure 3b shows the MRM detection of PFOA in a sample respectively.
Conclusions
Untargeted UHPLC–QToF followed by targeted UHPLC–MS/MS analyses were used to detect several PFAS in food containers collected randomly from local fastfood establishments from the region. The findings confirmed the growing body of research showing that PFAS can be found in food containers. The PFAS detected, such as PSPDA and N-EtPBSA, are rarely discussed in the literature, making their detection using a targeted approach very difficult due to the unavailability of standards. Continued research is needed on the prevalence of these uncommon PFAS, and the effects they may have on health. Furthermore, more untargeted testing in general is needed to identify the large and continuously changing body of PFAS used in consumer goods today.
Acknowledgments
This work was supported by the Center for Environmental Sciences & Engineering, Institute of the Environment at the University of Connecticut.
Conflict of Interest Statement
All authors do not have conflicts of interest.
References
(1) Glenn, G.; Shogren, R.; Jin, X.; Orts, W.; Hart-Cooper, W.; Olson , L. Per- and Polyfluoroalkyl Substances and Their Alternatives in Paper Food Packaging. Compr. Rev. Food Sci. 2021, 20, 2596– 2625. DOI: 10.1111/1541-4337.12726
(2) Begley, T. H.; White, K.; Honigfort, P.; Twaroski, M. L.; Neches, R.; Walker, R. A. Perfluorochemicals: Potential Sources of and Migration from Food Packaging. Food Addit. Contam. 2005, 22 (10), 1023–1031. DOI: 10.1080/02652030500183474
(3) Trier, X.; Granby, K.; Christensen, J. H. Polyfluorinated Surfactants (PFS) in Paper and Board Coatings for Food Packaging. Environ. Sci. Pollut. Res. 2021, 18, 1108–1120. DOI: 10.1007/s11356-010-0439-3
(4) Steenland, K.; Winquist, A. PFAS and Cancer, A Scoping Review of the Epidemiologic Evidence. Environ. Res. 2021, 194, 110690. DOI: 10.1016/j.envres.2020.110690
(5) Fact Sheet: 2010/2015 PFOA Stewardship Program. EPA, Environmental Protection Agency. www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet20102015-pfoa-stewardship-program (accessed 2021-04-15).
(6) “PFAS in Food: EFSA Assesses Risks and Sets Tolerable Intake.” European Food Safety Authority. www.efsa.europa.eu/en/news/pfas-food-efsa-assesses-risks-and-sets-tolerable-intake (accessed 2021-04-15).
(7) Genualdi, S. Analytical Methods for PFAS in Foods from FDA’s Total Diet Study Program., LCGC N. Am. LCGC: The PFAS Summit: A Virtual Symposium. Day 2, Session 1, 2022.
(8) Schaider, L. A.; Balan, S. A.; Blum, A.; Andrews, D. Q.; Strynar, M. J.; Dickinson, M. E.; Lunderberg, D. M.; Lang, J. R.; Peaslee, G. F. Fluorinated Compounds in U.S. Fast Food Packaging. Environ. Sci. Tech. Let. 2017, 4, 105‒111 including supporting information. DOI: 10.1021/acs.estlett.6b00435
(9) Susmann, H. P.; Schaider, L. A.; Rodgers, K. M.; Rudel, R. A. Dietary Habits Related to Food Packaging and Population Exposure to PFASs. Environ. Health Persp. 2019, 127 (10), EHP4092. DOI: 10.1289/EHP4092
(10) Blanco-Zubiaguirre, L.; Zabaleta, I.; Usobiaga, A.; Prieto,A.; Olivares, M.; Zuloaga, O.; Elizalde, M. P. Target and Suspect Screening of Substances Liable to Migrate from Food Contact Paper and Cardboard Materials Using Liquid Chromatography-High Resolution Tandem Mass Spectrometry. Talanta 2019, 208, 120394. DOI: 10.1016/j.talanta.2019.120394
(11) Zabaleta, I.; Negreira, N.; Bizkarguenaga, E.; Prieto, A.; Covaci, A.; Zuloaga, O. Screening and Identification of Per- and Polyfluoroalkyl Substances in Microwave Popcorn Bags. Food Chem. 2017, 230, 1977‒1992. DOI: 10.1016/j.foodchem.2017.03.074
(12) Vorst, K. L.; Saab, N.; Silva, P.; Curtzwiler, G.; Steketee, A. Risk Assessment of Per- and Polyfluoroalkyl Substances (PFAS) in Food: Symposium Proceedings. Trends Food Sci. Tech. 2021, 116, 1203‒1211. DOI: 10.1016/j.tifs.2021.05.038
(13) Li, D.; Zhang, Z.; Zhong, H.; Zhu, L.; Pan, J.; Zheng, J.; Lin, Q.; Liu, H. The Determination of Trace Per- and Polyfluoroalkyl Substances and Their Precursors Migrated into Food Simulants from Food Contact Materials by LC–MS/MS and GC–MS/MS. LCGC N. Am. 2019, 37 (7), 464‒475.
(14) Barhoumi, B.; Sander, S. G.; Tolosa, I. A Review on Per- and Polyfluorinated Aalkyl Substances (PFASs) in Microplastic and Food-Contact Materials. Environ. Res. 2022, 206, 112595. DOI: 10.1016/j.envres.2021.112595
(15) Ramirez Carnero, A.; Lestido-Cardama, A.; Vazquez Loureiro, P.; Barbosa-Pereira, L.; Rodriguez Bernaldo de Quiros, A.; Sendon, R. Presence of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) in Food Contact Materials (FCM) and Its Migration to Food. Foods 2021, 10 (7), 1443. DOI: 10.3390/foods10071443
(16) EPA537 Method, https://www.epa.gov/pfas/epa-pfas-drinking-water-laboratory-methods (accessed 2021-04-15).
About the Authors
Noah B. Liguori-Bills, James D. Stuart, Sarah A. Ayers, Christopher R. Perkins, and Anthony A. Provatas are with the Center for Environmental Sciences & Engineering, Institute of the Environment, at the University of Connecticut, Storrs, Connecticut. Direct correspondence to: anthony.provatas@uconn.edu

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.











