Demystifying Sample Preparation for Headspace Analysis Using Direct Injection Mass Spectrometry
Sample preparation for headspace analysis of volatile organic compounds (VOCs) by gas chromatography (GC) is well understood. Newer direct‑injection mass spectrometry (DIMS) techniques have found application in headspace analysis, but with some necessary modifications due to (i) the removal of the chromatographic column, and (ii) the soft chemical ionization that is used in these techniques. These factors place important constraints on the total load of VOCs—whether of solvents, analytes, or other VOCs in the matrix. This article summarizes the basic differences between DIMS and GC approaches, and describes the strategies by which headspace-DIMS can be successfully adopted.
Headspace analysis is widely used with gas chromatography (GC) for the analysis of volatile organic compounds (VOCs) in solution or solid matrices (1). In addition to the obvious requirements—that the sample be placed in a sample vial and headspace be developed—the GC technique itself can impose additional sample preparation requirements, including dry purging, derivatization of polar compounds, and headspace enrichment. These factors, coupled with the time it takes to separate the compounds in the column, can have a marked effect on sample throughput.
In recent years, direct sample analysis techniques have become available that provide high specificity without the need to utilize chromatographic separation. The most effective of these are based on direct‑injection mass spectrometry (DIMS), which use soft chemical ionization coupled with mass spectrometry (MS) to provide specific analysis. Where it is applicable to the matrix and analytes, DIMS methods can simplify sample preparation and increase sample throughput, providing lower cost per sample.
Using selected ion flow tube mass spectrometry (SIFT-MS; see [2]) as representative of DIMS techniques developed for gas-phase VOC analysis, this article describes the fundamental differences between DIMS and GC, and considers briefly how these impact on sample preparation requirements for headspace analysis using DIMS.
DIMS and Gas Chromatography: Key Differences
Elimination of the chromatographic column in DIMS techniques is the most obvious difference compared with GC and is addressed first. The second and more subtle point is that suitable ionization must be utilized to enable DIMS to be applied. Here, the principles are illustrated using the soft chemical ionization applied in SIFT-MS.
DIMS analysis is chromatography‑free. In DIMS techniques, the sample is continuously delivered to the ionization region of the instrument, so there is no means by which a solvent front or matrix VOCs can be switched out. This must be addressed through use of compatible (low reactivity) solvents and ionization agents—so-called reagent ions in SIFT-MS. This is addressed in the next section.
In GC, rapid headspace injection, whether from a pressurized sample loop, syringe, cryotrap, and so on, is used to provide the focused peak shape required for good chromatographic separation. This approach is ill-suited to DIMS techniques, where the best result is obtained when headspace is introduced into the DIMS instrument continuously at a steady flow rate for simultaneous ionization and detection of the analytes. To date, this has been most effectively achieved by integrating syringe‑injection autosamplers with SIFT-MS instruments, providing high repeatability even when no internal standard is utilized (2)—in contrast to the potentially poor repeatability when large headspace volumes are injected into a GC inlet liner. Figure 1 shows a headspace injection into a SIFT‑MS instrument, where the instrument is continuously analyzing sample during the slow, steady sample introduction. The figure shows the concentration generated as a function of time, with the reported value taken from the mean over the injection. Note that solid-phase microextraction (SPME) and similar headspace preconcentration approaches are not so well suited to DIMS due to small capacity and rapid sample desorption.
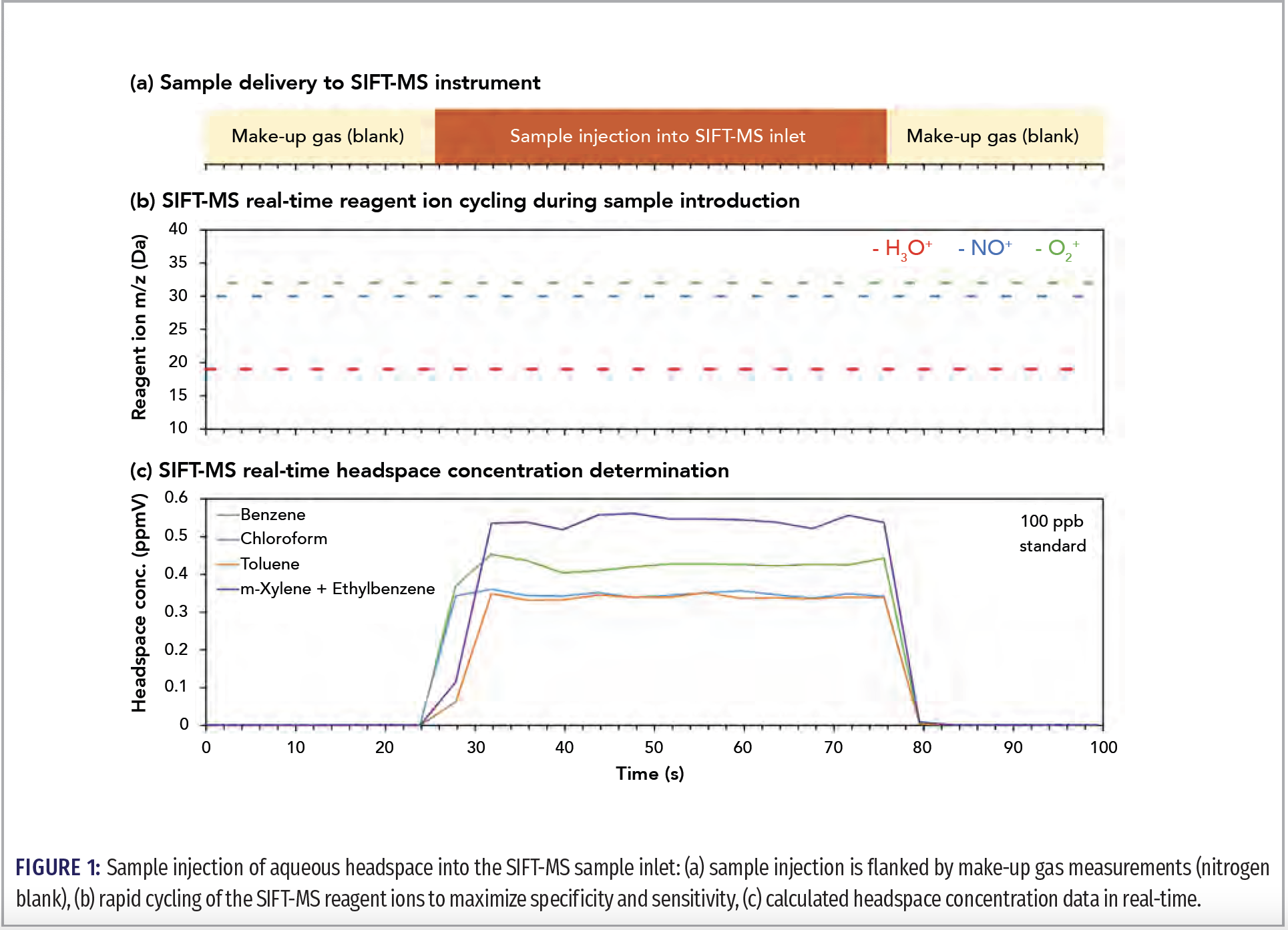
DIMS techniques with very soft ionization are most suitable. In DIMS instruments, removal of the chromatographic column shifts responsibility for achieving specific analysis entirely to the combined ionization and detection system. Since electron ionization (EI) is too harsh to provide specific analysis without chromatography, soft chemical ionization is typically utilized because minimal fragmentation occurs for most VOCs. In SIFT‑MS instruments, three positively charged reagent ions (H3O+, NO+, O2+) and up to five negatively charged reagent ions (OH-, O-, O2-, NO2, NO3-) are available. These reagent ions provide highly sensitive analysis of most VOCs, and they have very efficient ionization mechanisms, resulting in non-discriminatory sample analysis—even for underivatized volatile fatty acids. Analysis is achieved directly from air because the ions are “blind” to the bulk components, namely oxygen, nitrogen, argon, and carbon dioxide in positive ion mode, and they react only very slowly with water.
Specific analysis is obtained by the use of reagent ions that usually ionize a given VOC via different mechanisms—the relevance of a given mechanism being dependent on the functional group (3) and molecular weight of the VOC—and the fact that, uniquely in SIFT‑MS, these ions are switchable in real time (Figure 1b).
Matrix Compatibility and Solvent Selection
Continuous flow of sample through the DIMS instrument with no temporal preseparation of components creates some special challenges for transfer of methods from chromatographic techniques. The overarching principle that needs to be followed for quantitative analysis is that the bulk of the headspace must be non‑reactive—at least with one reagent ion. Although it varies based on composition and the relative sensitivities of each reagent ion, for commercial SIFT‑MS instruments a typical upper limit to the total headspace concentration for all reactive compounds is 100 parts‑per‑million by volume (ppmV). This headspace could be dominated by solvents and/or matrix compounds.
Solvents—whether used for extraction, derivatization, preconcentration, or in the creation of calibration standards—need to be selected with care in SIFT-MS. In contrast to GC, water is the preferred solvent for SIFT-MS because its reagent ion reactions are very slow (4). For organic solvents, at least one reagent ion must have significantly lower sensitivity to the solvent and/or the headspace partitioning of the solvent needs to be poor. Typically, an intermediate dilution step (in water) is also required (2). Work is currently underway to quantify the maximum proportion of organic solvent in water that headspace-SIFT-MS can accommodate under the standard headspace conditions described in Perkins and Langford (2021) (4). For dimethyl formamide (DMF), dimethyl sulfoxide (DMSO), methanol (using NO+ only), and triacetin, from 5 to 10% solvent in aqueous solution appears workable.
Assessment of the sample matrix takes a similar approach. If the headspace composition is not known—whether for solutions or the solid phase, such as polymers—untargeted analysis assists identification of matrix species and assessment of the impact that these will have on quantitative SIFT-MS analysis. If total concentrations are likely to exceed the SIFT‑MS instrument’s dynamic range, then dilution, for example, through slower headspace injection or dilution in aqueous, can be used. Clearly, limits of quantitation will be reduced proportionately.
Headspace Enrichment, Preconcentration, and Drying
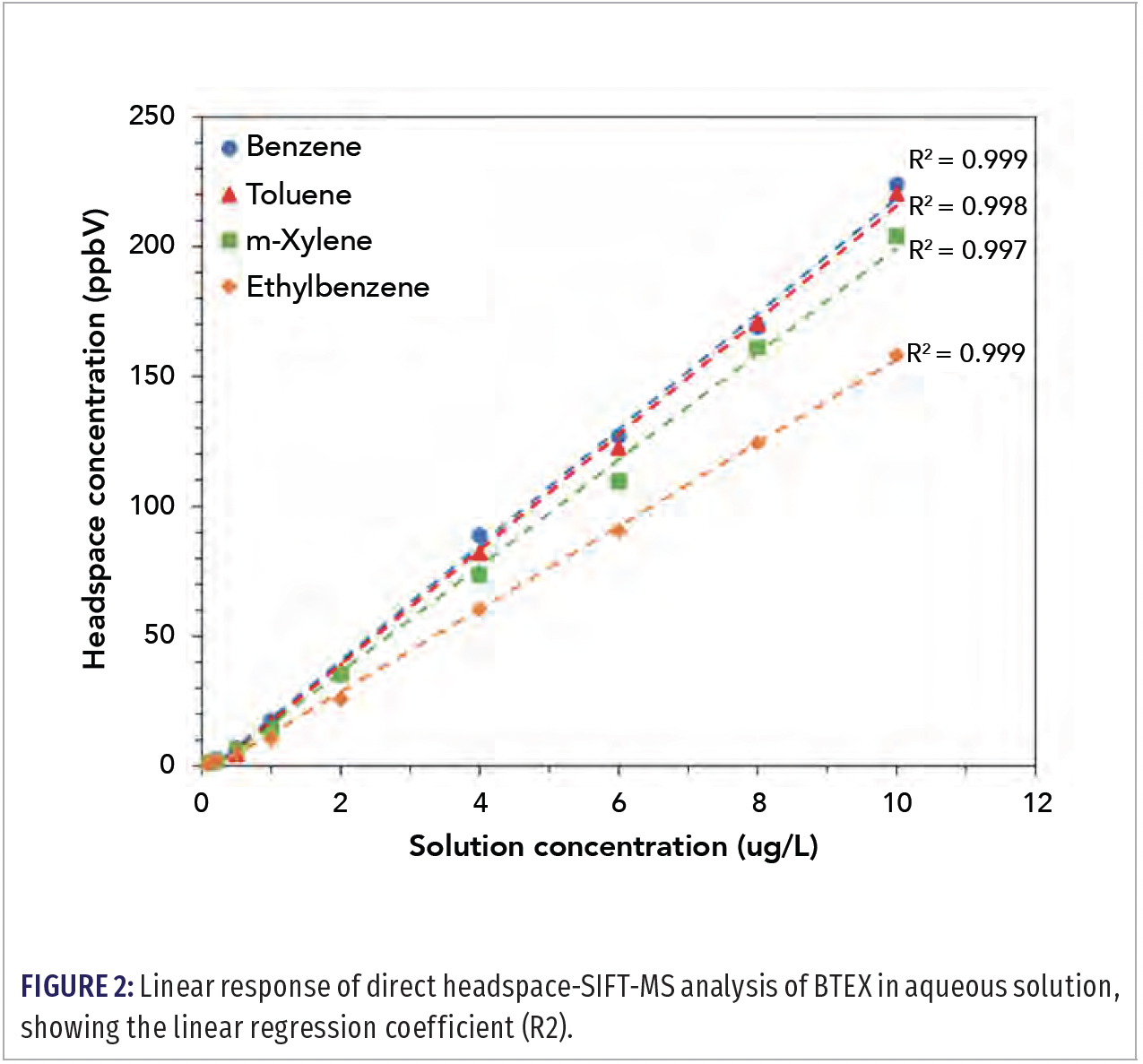
SIFT-MS quantifies VOCs to sub part‑per‑billion concentrations (by volume, ppbV) direct from headspace without preconcentration. Given the robustness of SIFT-MS analysis to water, analytes that partition readily to headspace from aqueous solution can usually be analyzed to low ppb concentrations in water without requiring dry purging, enrichment, or preconcentration. Hence the use of dynamic headspace analysis, purge-and-trap, or “workaround” approaches, such as dispersive liquid–liquid microextraction, can usually be avoided by implementing DIMS. Figure 2 shows linearity data obtained for benzene, toluene, ethylbenzene, and the xylenes (BTEX) measured directly from unmodified aqueous solution without the use of an internal standard (2). Relative standard deviations (RSDs) less than 10% were obtained over six replicates in the 1–9 μg/L concentration range in solution.
For compounds that partition poorly from aqueous solution, strategies used with GC are appropriate with the caveat that they must not generate compounds in headspace that react with reagent ions to an extent that compromises the dynamic range, nor interfere with target compounds. Options include salting of solution (4), modification of pH, use of surfactants, and use of a second solvent (similar to the previous section).
Derivatization
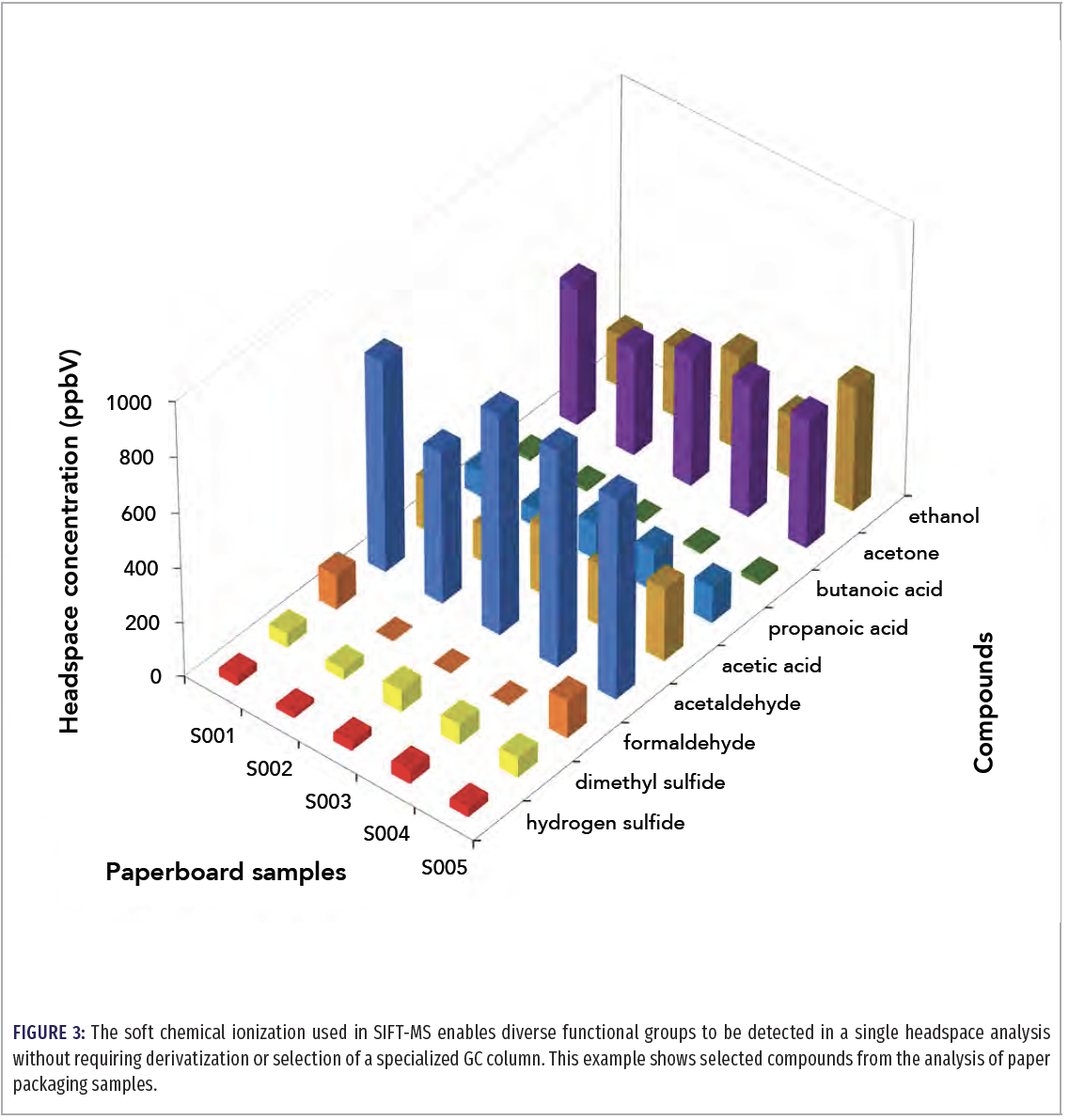
Analysis of very polar, low-molecular-weight volatiles is often a significant challenge for GC, requiring derivatization and/or use of a specialized column. For SIFT-MS, however, the elimination of the chromatographic column and the application of soft chemical ionization, with consistent sensitivity regardless of functional group, significantly simplifies preparation and analysis of these compounds. Figure 3 illustrates this benefit of SIFT-MS using paper packaging samples.
Although these compounds are readily ionized in the SIFT-MS flow tube, effectiveness of sample delivery needs to be assessed:
- Is the underivatized compound sufficiently volatile?
- Does it partition sufficiently to headspace?
- Is it prone to “sticking” to surfaces?
Generally, for low-molecular-weight acids, aldehydes, and amines, direct analysis can be achieved using suitable incubation coupled with heating and passivation of surfaces involved in headspace transfer to the instrument.
Conclusion
DIMS techniques such as SIFT-MS have the potential to increase sample throughput for headspace analysis through simplification of sample preparation and removal of the chromatographic column. The greatest reduction in sample preparation requirements compared to GC is realized for aqueous systems in which analytes partition well into the headspace. DIMS also simplifies both sample preparation and analysis of high polarity analytes, such as low-molecular-weight aldehydes, acids, and amines, as derivatization is not required. Furthermore, the high sensitivity of DIMS techniques typically means that a preconcentration step is unnecessary. Sensitivity can be reduced through the presence of reactive solvents or matrix species. For compatible matrices, throughputs of greater than 250 samples for each day are achieved.
References
(1) Kolb, B.; Ettre, L. S. Static Headspace-Gas Chromatography–Theory and Practice, 2nd ed.; John Wiley & Sons, 2006.
(2) Perkins, M. J.; Langford, V. S. Application of Routine Analysis Procedures to a Direct Mass Spectrometry Technique: Selected Ion Flow Tube Mass Spectrometry (SIFT-MS). Rev. Sep. Sci. 3 (2), e21003 (2021). DOI: 10.17145/rss.21.003
(3) Smith, D.; McEwan, M. J.; Španěl, P. Understanding Gas Phase Ion Chemistry Is the Key to Reliable Selected Ion Flow Tube-Mass Spectrometry Analyses. Anal. Chem. 92, 12750–12762 (2020). DOI: 10.1021/acs.analchem.0c03050
(4) Perkins, M. J.; Langford, V. S. Standard Validation Protocol for Selected Ion Flow Tube Mass Spectrometry Methods Applied to Direct Headspace Analysis of Aqueous Volatile Organic Compounds. Anal. Chem. 93, 8386–8392 (2021). DOI: 10.1021/acs.analchem.1c01310
Vaughan Langford is Principal Scientist at Syft Technologies in New Zealand. Mark Perkins is a senior applications chemist and SIFT-MS expert at the Laboratory Services Division of Element (formerly Anatune Limited), based in Cambridge, UK. Direct correspondence to: vaughan.langford@syft.com or mark.perkins@anatune.co.uk

Fundamentals of Benchtop GC–MS Data Analysis and Terminology
April 5th 2025In this installment, we will review the fundamental terminology and data analysis principles in benchtop GC–MS. We will compare the three modes of analysis—full scan, extracted ion chromatograms, and selected ion monitoring—and see how each is used for quantitative and quantitative analysis.











