Analysis of Boronic Compounds as Potential Mutagenic Impurities in Drug Substances By GC–MS
Bis(pinacolato)diboron (BPD) and tetrahydroxydiboron (BBA) are commonly used reagents in Suzuki-Miyaura coupling for the synthesis of many intermediates and active pharmaceutical ingredients (APIs). These reagents have tested Ames positive and are classified as mutagenic by the International Council for Harmonization (ICH), under the “Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk,” M7 (ICH M7) as Class 2 impurities. Because BPD and BBA are mutagenic, they need to be controlled and subsequently detected down to very low levels. Current methods using inductively coupled plasma–mass spectrometry (ICP-MS) give only the total boron content, which is problematic if the API also contains a boron molecule. In this paper, we describe a novel gas chromatography (GC)–MS approach for the low-level analysis of BPD and BBA in drug substances, even in the presence of boron from other sources. The analysis of BBA required derivatization of BPA to BPD, resulting in quantitation limits of 30 ppm of BBA (back calculated from the derivatized compound). BPD could be analyzed directly with quantitation limits of 2.5 ppm.
The Suzuki-Miyaura reaction forms a carbon–carbon bond, typically using a palladium catalyst, and has become routine in synthesizing pharmaceutical products (1). Bis(pinacolato)diboron (BPD) and tetrahydroxydiboron (BBA) are commonly used reagents in Suzuki-Miyaura coupling for synthesizing many intermediates and active pharmaceutical ingredients (APIs) (2). A recent publication by Hansen and others (3) reported that BPD and BBA tested positive in the Ames assay and are considered Class 2 impurities based on the International Conference on Harmonization (ICH) M7 “Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk” (4). As such, they should be closely monitored and controlled at or below acceptable limits (acceptable threshold of toxicological concern, or TTC) (4). As outlined in the guidance, acceptable total daily intakes for individual impurities for the duration of treatment greater than 10 years and between 1–10 years are less than 1.5 μg/day and 10 μg/day respectively, for an estimated maximum daily dose of 0.2 g (as is the case with the API studied). The corresponding acceptable TTC limits for BPD or BBA are 7.5 ppm and 50 ppm, respectively.
Previously, colorimetric methods have been used to determine low levels of boron (5), and Sah and Brown (6) have reviewed more modern techniques for determining boron content and the different boron isotopes. Atomic emission or absorption spectrometry have shown poor sensitivity. Prompt-γ spectrometry can calculate the boron concentration in solid samples, which makes it useful; however, it is not suitable for low levels of boron. Inductively coupled plasma–optical emission spectrometry (ICP-OES) and ICP–mass spectrometry (ICP-MS) have been used to analyze total boron content in APIs. ICP-MS is the more sensitive technique; however, it only quantifies the total boron in an API and cannot differentiate between a boron molecule in the structure of the API (if one exists) and impurities from the Suzuki-Miyaura coupling or any other boron-related impurities in the API (7). To have a more generic methodology, chromatographic quantitation, which can separate the boronic species, is desirable.
A high throughput analysis of boronic acids with aromatic groups has been reported by Pandiiyan (8) and used for reaction monitoring samples of Suzuki-Miyaura coupling. A lack of chromophores and poor ionization make it difficult to analyze BBA by high performance liquid chromatography (HPLC) or LC–MS, whereas a lack of solution stability under aqueous conditions makes it difficult to analyze BPD by LC–MS. However, synthetic chemists working with boronic compounds have been using a combination of gas chromatography (GC) and GC–MS to monitor chemical reactions (9–11).
To determine the feasibility of detecting the individual boron-containing compounds in the absence of any matrix or API, BBA and BPD were analyzed by GC with a flame ionization detector (FID) and GC–MS. For this portion of the method development, BBA and BPD were dissolved in an aprotic solvent (carbon tetrachloride) to avoid any decomposition of the components at low concentrations in the solution. The precision of direct injection GC improves when using an internal standard because of high flow rates and split ratios (12). A commercially available boronic compound with a similar structure and fragmentation pattern to BPD, bis(hexyleneglycolato)diboron (BHGD), proved to be a useful internal standard for quantifying BPD down to 0.09 μg/mL. Although BBA was not detected when analyzed by direct injection GC, its derivatization to BPD allows it to be detected by both FID and MS detectors, whereas BBA could not.
BPD was employed for the synthesis of API-A. The method development work in Part 1 was successful in developing a quantitative method for the BPD solution, which used carbon tetrachloride (CCl4) as the diluent. However, for this study, dimethyl sulfoxide (DMSO) was selected as the diluent because API-A was not soluble in CCl4. Using the results from Part 1, a method was developed for the quantification of BPD in the API-A matrix also using BHGD as an internal standard. The method detection limit achieved was 0.16 μg/mL of BPD.
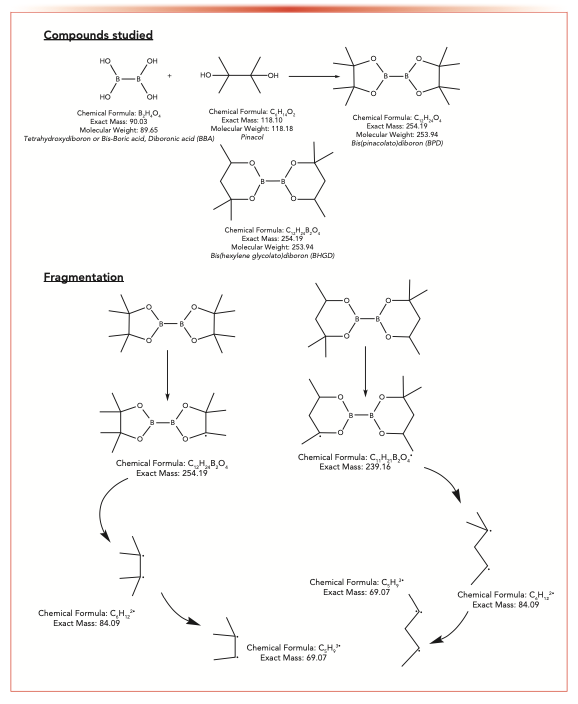
In contrast, API-B was synthesized using BBA. To improve the detection of BBA using GC–MS, BBA was derivatized to BPD using pinacol (Scheme 1). The amount of BBA in the API-B matrix was analyzed by GC–MS after BBA was derivatized to BPD and could be quantified down to a limit of 2.5 ppm. Because the final product (BPD) could be purchased, the efficiency of derivatization was able to be monitored.
SCHEME 1: Structures of compounds and studied fragments.
Materials and Methods
Part 1: Detection of BPD in CCl4 by GC–MS
All compounds (BBA, BPD, BHGD, and pinacol) for this study were sourced from Merck KGaA, with the exception of carbon tetrachloride (CCl4), which was purchased from Acros Organics (part of Thermo Fisher Scientific). GC–MS analyses were performed on an Agilent 7890B GC system coupled with a 5977A mass selective detector with an internal splitter (~1:10) to both a mass spectrometer (MS) and a flame ionization detector (FID) and Mass Hunter software. The GC separation was achieved on an Agilent HP-5MS having the dimensions 30 m x 0.25 mm and a film thickness of 0.25 μm.
For the GC program, high purity helium (Airgas) was used as a carrier gas with a constant flow of 2 mL/min. The column oven temperature was initially set at 80 oC, and held for 1 min, then raised at a rate of 2.5 oC/min to a final temperature of 250 oC and held for 1 min. The sample was injected using a 7683B auto sampler. The inlet temperature was held at 240 oC and operated in a split mode with a 10:1 split ratio. An ultra-inert deactivated inlet liner with glass wool for split injections (Agilent Part No. 5190-3163) was used. The retention times (tR) of the components studied are as follows: CCl4: 1.65 minutes, BPD: 5.49 min, and BHGD: 5.58 min.
The Agilent 7890N was interfaced with the mass spectrometer (operating in electron ionization, or EI, mode) by a direct transfer line maintained at 250 oC with the source temperature set at 230 oC. The mass spectrometer was operated in both total ion count (TIC) and single ion monitoring (SIM) modes. The SIM was set to scan for two ions at m/z 69.1 and 239.2 as these ions are common to both BPD and BHGD electron ionization fragmentation (see Scheme 1). It is noted that the fragments of interest are identical; however, because the compounds can be separated by chromatography, each of these compounds can be used as an internal standard for the other. By collecting the ion count chromatograms for the same ions, internal standard calculations were made. The ion at m/z 84.1 is also a strong ion for both of these compounds; however, CCl4 interferes with the signal so that ion could not be used in Part 1 of the study.
Part 2: Detection of BPD in API-DMSO Matrix by GC–MS
Analysis of BPD was conducted by GC–MS with BHGD as the internal standard. The chemicals used were anhydrous dimethyl sulfoxide (DMSO) (Merck KGaA), BPD (Aldrich, 473294-25G), and BHGD (Aldrich, 525685-1G). Anhydrous DMSO was further treated with magnesium sulfate as a drying agent in a ratio of 60–65 mg magnesium sulfate to 1 mL DMSO.
GC–MS analyses were performed on an Agilent 7890B GC system coupled with a 5977A mass selective detector. The GC separation used an identical column to the one used in Part 1.
For the GC program, high purity helium was used as a carrier gas with a constant flow of 2 mL/min. The column oven temperature was initially set at 80 oC, and held for 3 min, then raised at a rate of 15 oC/min to a final temperature of 250 oC and held for 5 min. The sample was injected using a 7683B auto sampler. The inlet temperature was held at 250 oC and operated in a split mode with 100:1 split ratio. An ultra-inert deactivated inlet liner with a glass wool injection and single taper (Agilent Part No. 5190-3163) was used.
The Agilent 7890N was interfaced with the mass spectrometer (operating in electron ionization, or EI, mode) by a direct transfer line maintained at 220 oC, and the source temperature was set at 230 oC. When the m/z was at 84.1 and 239.2, it was used to detect BPD samples. For this study, m/z 84.1 was selected to monitor BPD because it has better response than m/z 239.2. To detect the BHGD internal standard samples, m/z 69.1, m/z 83.1, and m/z 239.2 were used, and m/z 69.1 was selected to monitor BHGD because it is an unique ion to BHGD. The mass spectrometer was operated in single-ion-monitoring (SIM) mode and set to scan for the following four ions: m/z 69.1, 83.1, 84.1, and 239.2. Because DMSO was used as a diluent in this study, the m/z 84.1 peak (which is the most intense peak for both the sample and internal standard) could be used as there was no longer an interfering peak when using this diluent.
BBA Derivatization Procedure
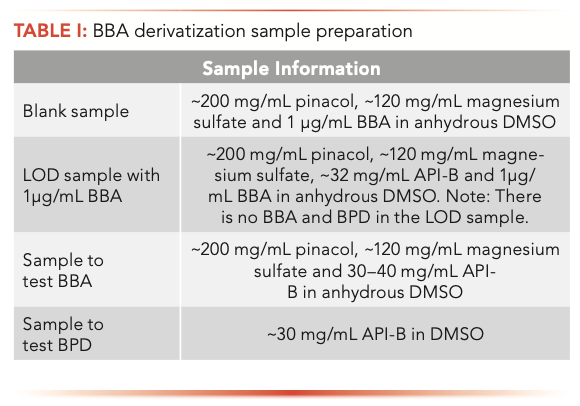
Because BBA was only used in the synthesis of API-B, the derivatization procedure was only used with API-B. The BBA in API-B was derivatized into BPD using pinacol by mixing approximately 40 mg of API-B, 200 mg of pinacol, and 120 mg of magnesium sulfate in 2 mL of anhydrous DMSO. The mixture was prepared in a scintillation vial and stirred periodically using a magnetic stirrer. Aliquots were taken between 2.5 and 19 h, centrifuged for 5 min, and then the supernatant was removed. The supernatant was diluted at a 1:1 ratio with anhydrous DMSO and analyzed by GC–MS using instrument parameters described above in “Materials and Methods: Part 2.” A blank sample was run to ensure no carryover. Detailed sample information and preparation details are listed in Table I.
To evaluate the derivatization time required for conversion of BBA to BPD, neat solutions of different BBA concentrations in DMSO were prepared: 10 mg/mL, 10 μg/mL, 1 μg/mL, and 0.1 μg/mL, and these solutions were derivatized with an excess of pinacol. The mass-to-charge ratio (m/z) 84.1 was used to monitor BPD formation (as it is the strongest ion signal and under these conditions there no interfering signal from the solvent). Table II summarizes the conversion data noting that the optimal time for conversion at 1 μg/mL concentration is dependent on BBA concentration.
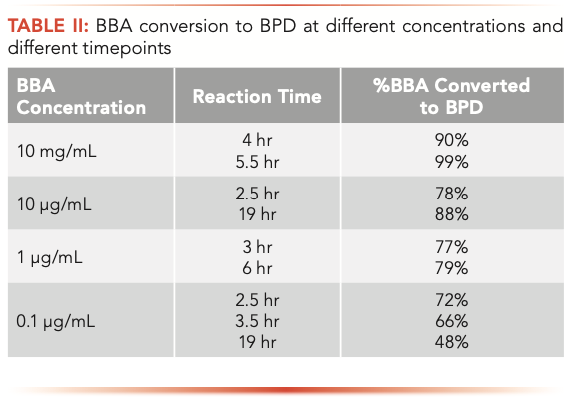
Based on the synthetic process, the endogenous BBA was likely negligible. A concentration of 1 μg/mL was tested to show feasibility of the derivatization of BBA in the API-B matrix. It took between 3 and 6 hours for about 80% of the 1 μg/mL BBA to be converted to BPD, which was tested against an authentic BPD sample. For verification of the method limit, the derivatized 1 μg/mL BBA sample was run as a process or system suitability sample prior to analyzing API-B samples.
Part 1: Results Determining the Limit of Detection of BPD With Internal Standard BHGD
The standard ionization technique for GC–MS is electronic ionization (or formerly electron impact), which has the advantage of always fragmenting molecules into the exact fragments every time. This process makes it difficult to detect parent compounds, but by monitoring only the fragments, GC–MS affords a sensitive technique for detecting small amounts of material in a matrix, which in this case, would be any number of active pharmaceutical ingredients (APIs). Scheme 1 shows the compounds investigated and the fragments monitored.
Collection of Data
Reproducibility with direct injection with GC has improved with the development of new instrumentation, but since these studies are looking to detect minute amounts of materials, an internal standard (BHGD) was used at a constant concentration of 4 μg/mL to minimize variability in injection.
Linear Range
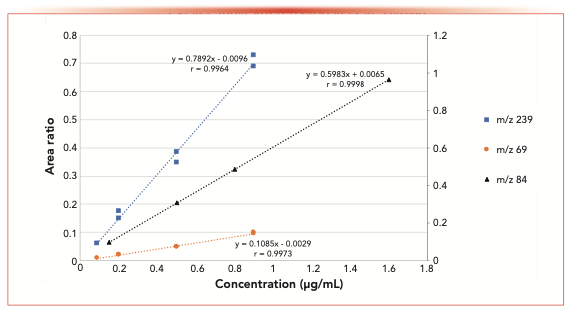
The sensitivity of using four different detection parameters (FID, MS, and SIM at m/z 69 and 239) was evaluated during this study. Because the carrier gas flow of the instrument post column was split to both the MS and the FID (split ~1:10), detection at both detectors was recorded almost simultaneously on the same sample for each data point. Figure 1 shows the concentration range of BPD studied and the resulting total ion count (TIC) and FID signals using BHGD as the internal standard. The resulting area ratios are plotted (BPD/BHGD). Interestingly, the slopes of the detectors are similar; however, the limit of detection for these two detectors using the split column flow was determined to be approximately 0.2 μg/mL. Figure 2 shows the analysis of similar samples, collecting the data by single ion monitoring (SIM) ions at m/z 69 or 239, to increase sensitivity. By monitoring only the extracted ions, the limit of detection was 0.09 μg/mL. The slope of the m/z 69 fragment curve is shallower than that of the TIC and FID, and the slope of the m/z 239 fragment curve is twice that of the TIC and FID. The fragment curve being this way is because of the response of the internal standard. When the total signal is used (for example in the FID and TIC) the response of the entire internal standard is similar to the entire sample and the slopes are similar. However, when using the SIM, only a portion of the signal is collected. The intensity of the m/z 69 signal is stronger in the internal standard than in the sample, making for very low ratios that change only slightly over the concentration range. The intensity of the m/z 239 signal in the internal standard is weaker but similar to that of the sample, which generated higher ratios and an overall stronger response at different concentrations, thereby generating a steeper slope. Here, the single ion scan that showed a similar intensity for both the internal standard and the sample, was the ion that gave a more discriminating response. Therefore, when choosing an ion to monitor, not only the intensity of that ion in the analyte needs to be considered, but also that of the internal standard as the optimum ion may not be the most intense one.
FIGURE 1: Area ratio response for FID and MS total ion count (TIC). BPD/BHGD area ratios showing linearity with detection by both MS TIC and flame ionization detector (FID).
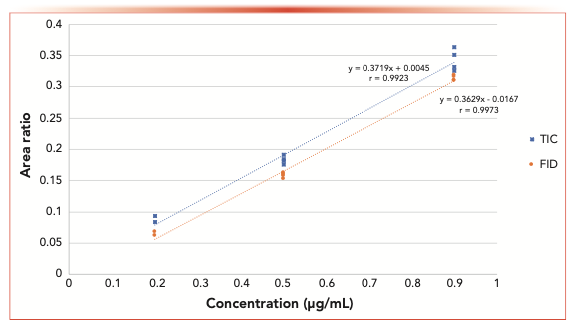
FIGURE 2: Area ratio response for SIM m/z 239 and m/z 69 in CCl4 and m/z 84 in DMSO. BPD/BHGD area ratios showing linearity using both m/z 69 and 239 fragment ions in CCl4 and the linearity of m/z 84 fragment in DMSO.
Reproducibility
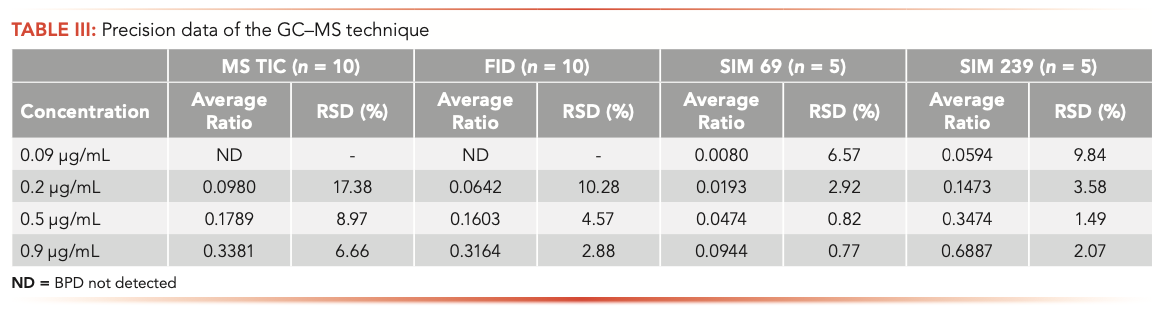
Reproducibility with direct injection can be a problem, therefore use of internal standards is common. Reproducibility was tested at multiple concentrations of BPD, while holding the internal standard (BHGD) concentration at approximately 4.0 μg/mL. Reproducibility was tested using MS TIC, FID detectors, and single ion monitoring (SIM) at m/z 69 and 239. At the lower levels, the relative standard deviations (RSDs) are less than 10% for the SIMs, which is consistent with typical assay methods for samples at the limit of detection (LOD). At 0.9 μg/mL, the RSDs for the SIMs and the FID are less than 3%. Table III summarizes, the reproducibility of the method at various concentrations with all four detection techniques.
Data Analysis
Because these samples were not in an API matrix, recovery was not tested. The GC–MS approach using SIM for detection was shown to be feasible and sensitive. As a next step detection in matrix was investigated. Because of the nature of the matrix, some modifications to the method and derivatization were necessary, such as a change in diluent to one in which the APIs studied are soluble.
It was found that there was little injection-to-injection and day-to-day variability in the signal both from the analyte and the internal standard. However, during the course of this study, there were cases where a series of injections showed an overall higher or lower signal for the analyte than the majority of the samples for that run. In each case, the signal from the internal standard varied in the same manner, which resulted in a constant ratio in line with other samples. It is proposed that eventually the need for the internal standard may be eliminated by having well-maintained equipment and tight system suitability controls. However, for the initial method setup, using the internal standard reduces the possibility of variability from injection to injection, allowing the focus of development to be on the sensitivity of the method.
Part 2a: Results of BPD Injection with Internal Standard BHGD in API-A
Collection of Data
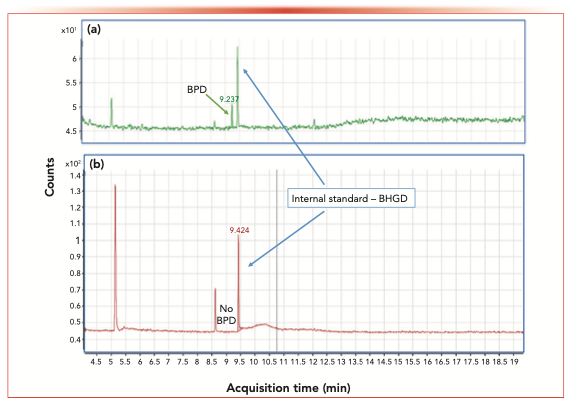
For the in-matrix study, the internal standard (BHGD) concentration was set to 3 μg/mL, and the monitoring ion used was m/z 84 because the interference from CCl4 was eliminated because of the use of DMSO as the diluent. The linearity of BPD was evaluated from 0.16 μg/mL to 3.2 μg/mL, resulting in a r value of 0.9998. Figure 2 shows a typical linearity curve plotting the area response ratios of BPD compared to BHGD. The LOD was found to be 0.16 μg/mL, and the LOQ was 0.48 μg/mL. The RSD of the BPD LOQ of six injections was 4.3%, showing good reproducibility at low levels. Figure 3a shows a typical chromatogram of BPD (with the corresponding internal standard) at the LOD concentration.
FIGURE 3: Counts vs. acquisition time (in min) for (a) Chromatogram of LOD standard using SIM m/z 84.1 (BPD concentration 0.15 μg/mL vs. internal standard), and (b) Chromatogram of API-A sample spiked with internal standard BHGD at RT 9.42 min, with no BPD detected at tR 9.23 min in API-A.
To evaluate the recovery of BPD from the matrix at the LOQ level, 0.48 μg/mL of BPD was spiked into API-A. The average recovery was 119%, and the RSD of the three spiked samples was 4.7%. A LOD of BPD at 0.16 μg/mL corresponds to 40 ppm of BPD in API-A.
Data Analysis
The following parameters were evaluated: specificity; system precision; accuracy; method precision; linearity; LOQ; and LOD. This method is considered fit for the purpose to determine BPD in API-A.
Seven batches of API-A have been analyzed using this method and no BPD has been detected in any of the batches. A typical chromatogram is shown in Figure 3b (LOD <0.16 μg/mL, corresponding to <40 ppm in API-A).
Part 2b: Results of BBA Detection Using a Derivatization Method
Previous studies have shown that in the absence of a matrix (Part 1) and in a drug substance matrix (Part 2a), and using an internal standard, direct injection, and MS detection, BPD can be detected down to low levels, such as 2.5 ppm.
Collection of Data
A second matrix, API-B, was tested for both BPD and BBA. The range of BPD (prior to derivatization of the BBA in the matrix) in API-B was evaluated from 0.0625 μg/mL to 1 μg/mL, yielding a r value of 0.9993 using polynomial regression. The LOD of the method is 0.0625 μg/mL, and the LOQ of the method is 0.25 μg/mL. The RSDs of the BPD LOQ of the six injections was 9.8%. The LOD of 0.0625 μg/mL corresponds to 2.5 ppm BPD in API-B.
Data Analysis
Using the derivatization method presented to convert BBA to BPD, the amount of BBA in matrix API-B was analyzed. The BBA level of 1 μg/mL corresponding to approximately 32 ppm of BBA in API-B was selected as the limit for the samples, since the acceptable daily intake for API-B is less than 50 ppm, which is the calculated TTC limit. A limit of 0.1 μg/mL of BBA corresponding to 3.2 ppm of BBA in API-B would be needed if the acceptable daily intake of BBA is required to be lower than 5 ppm. This level was shown to be achievable.
This test is considered sufficient to determine BBA to a limit of 32 ppm and BPD to 2.5 ppm in API-B.
Conclusion
This article summarizes the results of several iterations developing a method to determine the levels of several boronic species resulting from Suzuki-Miyaura coupling. Linearity and limits of detection were established for both neat compounds and compounds in matrix. Direct analysis of BPD using GC–MS and an internal standard, BGHD, was successful both in and out of an API matrix. BPD could be detected in matrix down to 2.5 ppm. The boronic compound, BBA, however, required derivatization prior to similar analysis by GC–MS. Detection of derivatized BBA in matrix was successful down to levels less than 32 ppm.
As more scrutiny is placed upon genotoxic impurities, tests for these boronic impurities will become more common. Because derivatization is sometimes required, it would be worth investigating the automation of the derivatization to further streamline these analyses.
Acknowledgment
The authors would like to thank Jonathon Truong for providing the derivatization conditions.
References
(1) J. Magano and J.R. Dunetz, Chem. Rev. 111(3), 2177–2250 (2011).
(2) N. Miyaura, and A. Suzuki, Chem. Rev. 95, 2457−2483 (1995).
(3) M.M. Hansen, R.A. Jolly, and R.J. Linder, Org. Process Res. Dev. 19, 1507–1516 (2015).
(4) International Conference on Harmonization, ICH M7(R1), Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk (ICH, Geneva, Switzerland, 2018).
(5) F.J. Lopez, E. Gimenez, and F. Hernandez, Fres. J. Anal. Chem. 346(10–11), 984–987 (1993).
(6) R.N. Sah and R.H. Brown, Microchem. J. 56(3), 285–304 (1997).
(7) I. Patel, C.J. Venkatramani, A. Stumpf, L. Wigman, and P. Yehl, Org. Process Res. Dev. 21(2), 182–186 (2017).
(8) P.J. Pandiyan, R. Appadurai, and S. Ramesh, Anal. Methods 5(13), 3386–3394 (2012).
(9) A. Bonet, C. Sole, H. Gulyás, and E. Fernándex, Org. Biomol. Chem. 10, 6621–6623 (2012).
(10) C. Pubill-Ulldemolins, A. Bonet, H. Gulyás, C. Bo, and E. Fernándex, Org. Biomol. Chem. 10, 9677–9682 (2010).
(11) S.K. Bose and T.M. Marder, Org. Lett. 16, 4562–4565 (2014).
(12) G.K. Webster and L. Kott, Eds., Chromatographic Method Development: Chapter 12, Considerations for Using GC in the Pharmaceutical Setting (Pan Stanford, Singapore, 2020).
Zhisong Ji is with Millennium Pharmaceuticals, Inc., in Cambridge, Massachusetts. Jelena Petrovic is with Ikena Oncology in Boston, Massachusetts. Laila Kott is with Xenon Pharmaceuticals Inc. in Burnaby, British Columbia, Canada. Direct correspondence to: lkott@xenon-pharma.com.

Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.
Fundamentals of Benchtop GC–MS Data Analysis and Terminology
April 5th 2025In this installment, we will review the fundamental terminology and data analysis principles in benchtop GC–MS. We will compare the three modes of analysis—full scan, extracted ion chromatograms, and selected ion monitoring—and see how each is used for quantitative and quantitative analysis.