Advanced Topics in Solid-Phase Extraction: Chemistries
LCGC North America
Although the majority of solid-phase extraction (SPE) is performed with conventional bonded silica- and polymeric-phases, difficult and complex samples may require more specialized stationary phases. In this installment of "Sample Prep Perspectives," columnist Ron Majors discusses advanced topics such as multimodal SPE, restricted-access media, molecular imprinted polymers, immunoaffinity extraction phases, and other class- or compound-specific sorbents. These phases provide additional selectivity, and procedures using them can be automated. Representative applications will be presented.
Over the years, "Sample Prep Perspectives" columns by myself and invited guest authors have covered various aspects of solid-phase extraction (SPE) including the basics (1–7), applications and method development (8–12), automation (13–16), dedicated symposia (17,18) and special supplements (19). Today, SPE remains one of the more important sample preparation techniques for chromatography and has branched out into other areas by employing new formats, new chemistries via novel sorption and partition mechanisms, multidimensional techniques, and miniaturized systems. This installment of "Sample Prep Perspectives" will review some of these advances in newer phase chemistries that might be directly applicable to solving your everyday sample preparation problems, especially when dealing with complex mixtures. I will divide my coverage into different categories that employ related concepts.

Ronald E. Majors
Quick Review of the Basics of SPE
SPE is a sample preparation technique based upon principles similar to those of high performance liquid chromatography (HPLC). It is used for the selective sorption of analytes or interferences from simple to complex matrices. It is used for sample cleanup and analyte concentration preceding HPLC, gas chromatography (GC), ion chromatography, and other separation techniques. It has replaced many of the classical liquid–liquid extraction (LLE) techniques. Relative to LLE, SPE uses much less solvent, improves sample throughput, provides more tunable selectivity by appropriate choice of stationary phase, is more readily automated, and avoids the formation of emulsions. In many cases, SPE provides cleaner extracts and provides higher and more reproducible recoveries.
In its simplest form, SPE uses a packing material such as bonded silica (40–50 μm) or polymeric media packed into a plastic, medical-grade syringe barrel. Similar to an HPLC column, porous polymeric or metallic frits contain the packing in the cartridge format. Other popular SPE formats are disks, pipette tips, and 96-well plates. The stationary phases typically used in SPE cartridges are reversed-phase (C8, C18), ion-exchange (strong anion and strong cation exchange), or normal-phase (silica, cyano, amino) packings. Typically, there are four steps in SPE operation: conditioning, sample addition, washing, and elution (Figure 1). The conditioning step solvates the bonded phase so that it can readily accept the liquid sample load, the washing step removes interferences, and the elution step involves the use of a strong solvent to elute the analyte of interest in a small volume for direct injection into chromatographic column. Sometimes, the eluent is blown down by solvent evaporation to further concentrate the analyte or to allow redissolution of the analyte in a solvent more compatible with the subsequent chromatographic technique.
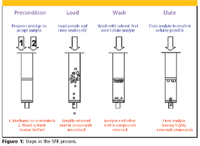
Figure 1
More and more applications are going to on-line or automated SPE. Compared with manual methods, automated SPE is less labor intensive, relieves the tedium of manual operation, requires less sample handling providing better recovery, is more reproducible, is performed in a closed system (less chance of sample oxidation or solvent evaporation), and allows smaller samples to be transferred and accommodated more easily. There are several approaches used for automation. The SPE phase can be packed into a stainless steel column and used repeatedly via column switching. Some SPE phases are packed into special high-pressure cartridges and used in an automated sampling device. Other forms of automation involve SPE cartridges, 96-well SPE plates, disks, or pipette tips that are handled by robotic or xyz liquid-handling devices. In these approaches, the sample is sometimes exposed to the atmosphere during handling.
Multimodal and Mixed-Phase Extractions
Most SPE sample preparation involves the use of a single mechanism (for example, reversed phase) and a single SPE device (for example, a cartridge). However, when more than one type of analyte is of interest or if additional selectivity is needed to isolate a single analyte, multimodal SPE can prove useful. Multimodal SPE refers to the intentional use of more than one retention mechanism. Experimentally, there are two approaches to multimodal SPE. Figure 2a depicts the serial approach, where two (or more) SPE cartridges are connected in series. For example, if one desired to isolate both hydrophobic organic molecules and inorganic cations, a reversed-phase cartridge (C18) could be placed on top of a strong cation cartridge (SO32– ). Conditions could be adjusted so that the organic molecules would be sorbed onto the C18 while the cations pass through the upper cartridge unretained. However, when they reach the strong cation exchange cartridge, they are strongly retained by ionic forces. The two cartridges can be separated and further treated to elute the respective isolates.
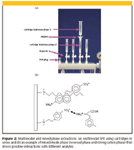
Figure 2
A second approach to multimodal SPE is shown in Figure 2b. Here a single cartridge could possess two (or more) functional groups to retain multiple species. Another possibility is that the two functional groups could interact with different portions of the same molecule. In Figure 2b, two functional groups (one C8 and one phenyl sulfonic) are bonded to the same substrate. In some cases, separate packings are blended and packed into the same cartridge. One popular application of multimodal SPE is the isolation of drugs of abuse and other pharmaceuticals from biological fluids (20). Koole and colleagues (21) used multimodal SPE for the multiresidue analysis of beta-agonists in human and calf urine. Their mixed-mode SPE cartridge had both C8 and cation-exchange functional groups.
The layered sorbent technique, described by Raisglid and Burke (22), is another version of multimodal SPE. In this approach, two (or more) different adsorbents are used to isolate differing molecular species. They used layered amino sorbent over a C18 sorbent to remove humic acids when removing pesticides from water samples. The amino sorbent strongly bound the humic molecules that have acidic or phenolic groups while the pesticides passed through the amino layer and were sorbed by the hydrophobic C18 layer. The pesticides could be eluted with an organic solvent from the C18 layer while the humic substances remained bound on the amino layer.
Restricted Access Media
Restricted access media (RAM) are a special class of SPE sorbents used for the direct injection of biological fluids such as plasma, serum, or blood. They are used most often for the analysis of small drugs, their impurities, and metabolites. The technology was discussed earlier by Boos and Randolphi (23,24) and reviewed recently by Cassiano and colleagues (25). Over the years, there have been many variations of these sorbents described as internal-surface reversed-phases, shielded hydrophobic phases, semipermeable surfaces, dual-zone phases, and mixed functional phases. For more information on the various classifications of RAM, consult reference 23.
The RAM phases that are the most popular are the dual-mode porous packings that are characterized by an outer hydrophilic layer and an inner surface porosity with a hydrophobic bonded phase. The outer hydrophilic surface with minimal interaction with proteins combined with small pores of the packing that exclude them cause the proteins to be eluted unretained, while small drugs and drug metabolites pass into the pores and are retained by hydrophobic interactions with alkyl bonded phases. Despite being described as "nonfouling" phases, RAM have had a reputation for eventual fouling with repeated injection of straight biological fluids. However, if the pH and organic solvent composition of the mobile phase are not optimized, protein precipitation can occur in the RAM causing fouling, so some care must be exercised in their use.
The RAM phases can be used in the single-column mode or with multidimensional LC–LC. In the single-column mode, conditions are selected to first exclude plasma proteins, then running gradient elution to elute and separate the drug compounds. Although this approach has worked satisfactorily, due to increased chances of fouling the RAM due to lack of reequilibration after gradient elution or due to inadequate selectivity of the hydrophobic stationary phase inside the pores, multidimensional LC–LC approaches have proven to be more popular. Here an additional reversed-phase column is plumbed into the system via a 6- or 10-port switching valve, isocratic conditions are used to inject the plasma onto the RAM, and an additional gradient pumping system is employed. In this approach, the trapped drug molecules from the RAM column are backflushed into a longer reversed-phase column, and gradient elution is performed to separate the impurities and metabolites. When multidimensional approaches are employed, the RAM column is backflushed and regenerated after each analysis. Long-term stability with repeated usage of the RAM column has been reported for soy isoflavones in rat serum (26). In addition, the reversed-phase column also has a longer life because plasma proteins are not injected onto this second column but are vented to waste.
Chang and colleagues (27) employed an on-line RAM-SPE column in removing protein and polar endogenous compounds from a human adrenocortical cell culture line H295 and measured anabolic steroids cleanly using reversed-phase LC–MS-MS. A column-switching valve was used with an ADS LiChrospher RP-4 RAM column (Merck, Darmstadt, Germany). The RAM phase consists of an outer diol layer and a C4 hydrophobic inner layer. Analytes backflushed from the RAM column were separated on a C18 column using gradient elution. Figure 3 shows three total ion chromatograms: a blank cell culture after removal of proteins and hydrophilic substances by the RAM column, a blank cell culture medium spiked with steroid analyte standards, and a cell culture medium of H295 cells treated with cAMP. The on-line RAM-LC procedure allowed the direct measurement of sample solutions without the time-consuming sample preparation procedure that the authors had used in the past. In addition, their on-line method showed better precision and accuracy compared with the radioimmunoassay methods previously used.
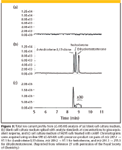
Figure 3
Molecular Imprinted Polymers
The molecular imprinted polymers (MIPs) are among the most selective phases used in SPE. The technique is sometimes referred to as molecularly imprinted SPE. Molecular imprinting is a technique that has been used in areas where selective recognition is required for complex separations or sample cleanup. An earlier article in LCGC (28) outlined the basics of MIP technology. Review articles (29–32) and a recent book (33) provide detailed information on the use and potential of MIPs in sample preparation and drug discovery.
A molecular imprinted polymer is a highly stable polymer that possesses recognition sites that are adapted to the three-dimensional shape and functionalities of an analyte of interest. The most common approach through noncovalent imprinting involves the use of a print molecule (template) that is chemically coupled with one of the building blocks of a polymer. After polymerization, the resulting bond must be cleaved to obtain a free selective binding site (receptor). The synthesis process is shown schematically in Figure 4. The selective interactions between the template and the monomers are based upon hydrogen bonding, ionic, or hydrophobic interactions. The most often used monomers are based upon methacrylic acid or methacrylates. The basic idea of MIPs is the "lock-and-key" concept, where a selective receptor or cavity on the surface of a polymer perfectly fits the template analyte that was used to prepare the MIP. The receptor site is complementary to the template in terms of its size, shape, and functionality. The concept is similar to immunoaffinity SPE phases, but obtaining and linking a suitable antibody for these sorbents can be very time consuming.
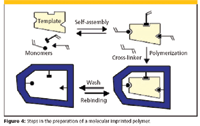
Figure 4
The removal of the template from the polymeric MIP is important not only to make available the interaction sites for increased sample capacity but also to ensure that the analyte to be isolated can be measured quantitatively. The lack of removal of the template molecules, even with exhaustive extraction, is one of the main problems with the acceptance of MIPs. The template molecules frequently bleed, sometimes give baseline drifts, and often interfere with the quantitation of the desired analyte, especially at low levels. One approach to overcome this limitation is to use a template that is similar to the analyte of interest. An example would be to use a brominated analog template rather than a chlorinated molecule of interest. If the analog can be separated from the analyte of interest, then the MIP will function as desired.
With aqueous mobile phases, MIPs can display reversed-phase and ion-exchange interaction because selective polar interactions are impaired. The MIP phases show the greatest selectivity when the experimental conditions are chosen that generate the selective interactions that are usually obtained in organic solvents used for the MIP synthesis. This approach allows the MIP to be used for trapping analytes from aqueous solution by hydrophobic or ionic interactions, then washed with a solvent that breaks selective binding of matrix components, and finally washed with an organic solvent that disrupts the strong bonds between the analyte and the MIP polymer matrix.
Because the SPE packing material is a polymer, depending upon the degree of crosslinking, there can be some swelling or shrinkage with a change in solvent. Such a physical change can modify the size of the receptor and change the selectivity of the MIP for the target analyte. In this regard, perhaps the synthesis of molecular imprinted organic–inorganic hybrid polymers (34) generates a more rigid substructure that does not swell and shrink.
A disadvantage of the MIP approach to SPE is the fact that each sorbent must be custom made. The analyst determines the specificity of the MIP by choosing the appropriate template molecule. The MIP can be synthesized in the laboratory using published procedures or the template molecule can be sent to a specialty laboratory that will prepare it. There are a number of companies that prepare custom MIPs, including Affinity Chromatography, Ltd. (Ballasala, Isle of Man, UK), Aspira Biosystems, Inc.(San Francisco, California), Ellipsa AG (Berlin, Germany), Instruction AG (Ludwigshafen, Germany), and MIP Technologies (Lund, Sweden). Because of the relatively long process involved in making MIPs for SPE, one can justify it only if the application will be required frequently or if there is no other way to perform sample cleanup.
Recently, one of the MIP synthesis companies, MIP Technologies, has begun to supply off-the-shelf MIPs. These standard MIP phases have been designed for specific analytes that are popularly encountered in complex matrices. Among those currently available in their standard MIP4SPE product line are sorbents optimized for
- clenbuterol in biological fluids;
- beta agonists, multiresidue extractions in urine and tissue samples;
- NNAL (4-methylnitrosamino-1-[3-pyridyl]-1-butanol), tobacco-specific nitrosamine in biological matrices;
- riboflavin (vitamin B2) in aqueous samples;
- triazines, multiresidue extraction in water, soil and food products;
- chloramphenicol , antibiotic in biological matrices; and
- beta blockers, multiresidue extractions in water and biological samples.
Besides molecularly imprinted SPE, MIPs are being exploited in many applications that include "tailor-made" separation materials, such as antibody–receptor binding site mimics, enzyme mimics in catalysis, and recognition elements in biosensors. There have been numerous applications of molecularly imprinted SPE for the successful isolation of target analytes in various matrices. Most of these applications resulted from "homemade" MIPs but point out the potential of these very selective phases in sample preparation experiments. Popular areas of applications have been in pharmaceuticals, particularly drugs in biological fluids, in food chemistry for pesticides, and in environmental chemistry for trace analysis. Schirmer and Meisel (35) synthesized an MIP for the selective SPE of chloramphenicol from honey. By adjustment of the methanol-water ratio in the wash step, matrix components from the honey could be removed and chloramphenicol could be nearly quantitatively extracted and the cleanup reduced matrix effects in their LC–MS-MS analysis. Andersson and colleagues (36) investigated the extraction of local anesthetics from human plasma using an MIP with a structural analog to their target molecules. They compared their MIP phases with a control (nonimprinted) polymer phase prepared without the template being present. To cut down on nonspecific sorption, some elaborate washing steps and additives were required during the extraction and elution conditions. They were able to demonstrate strong selective interactions between the analgesics and the MIP but were not able to eliminate template bleed totally despite using harsh extraction conditions that caused some polymer degradation. Martin and colleagues (37) synthesized a propranolol-specific MIP and also found that they had to work hard on obtaining the optimum conditions for binding the propranolol . They also observed nonspecific interactions between molecules structurally similar and dissimilar to the template molecule. The workers concluded that such molecular imprinted phases do have advantages over conventional SPE. Finally, Chapuis and colleagues (38) studied retention mechanisms of analytes in molecularly imprinted SPE. They investigated the extractions of triazines from grape juice and soil and found a high degree of selectivity for the target molecules compared to other compounds that possessed the same polarity and molecular size as the triazines. Molecular modeling provided a better understanding of the retention mechanism involved and was a useful guide for reducing nonspecific interactions.
One application area that has promise is the synthesis of chiral MIPs for the separation of enantiomers. Ever since the early work of Curti and Columbo (39), reported in 1952, on enriching racemic camphorsulfonic and mandelic acids using "tailored" silica gel adsorbents, there has been interest in the preparation of chiral MIPs. Here, the polymer is synthesized in the presence of one racemate and then used to separate a racemic mixture. Such packing would be useful for the quick separation of racemic mixtures in the pharmaceutical industry and could possibly be scaled up for large-scale processes. A number of successes in the chiral field have been achieved. Figure 5 shows an optimized LC separation of naproxen enantiomers on an (S)-naproxen MIP in a phosphate buffer-acetonitrile eluent. As expected, the more strongly retained S-enantiomer, which has stronger interactions with its surface receptor, is eluted last (40). This entire field of chiral MIPs was reviewed by Sellergren (41) but no reported commercial products have surfaced since this article was written.
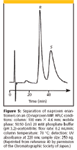
Figure 5
Immunoaffinity Extraction of Small Molecules
Similar to MIPs, immunoaffinity phases are based upon molecular recognition but use chemically attached mono- or polyclonal antibodies rather than surface cavities. Undoubtedly, the immunoaffinity phases are the most selective because they are designed primarily around biological antigen–antibody interactions that provide high selectivity and high affinity. These sorbents enable the selective extraction and concentration of individual compounds or classes of compounds from matrices, often in a single step. Antibodies for large biomolecules are readily available and have been used for many years in immunology and medical research and in the immunoextraction of enzymes, hormones and other biospecies. However, antibodies for small molecules are more difficult to obtain so the development of small molecule immunoaffinity extraction is more recent and less developed. Some excellent review articles are available for those who would like to understand immunoaffinity extractions in more detail (42–46).
As long as an antibody can be prepared, the numbers of immunoaffinity extraction sorbents can be almost unlimited. However, there is a great deal of time involved in making an antibody, purifying it, and bonding it to a solid support, so it only makes sense to develop immunoaffinity phases with a widespread use based upon popular groups of compounds that need to be isolated for further sample workup. Despite these limitations, there are several commercial products based upon immunoaffinity principles that have become available. Class-specific immunosorbents are available for a variety of pharmaceutical, food, and environmental applications. The Venture immunoaffinity SPE and column products of Grace Vydac (Columbia, Maryland) were introduced last year. These immunosorbents for food analysis include those dedicated to aflatoxins, lactoferrin, and vitamin B12. Steroid hormones phases for testosterone and nortestosterone and 17-β-estradiol and 17-α-ethynyl estadiol are available. For environmental pollutants, phases for chlorophenoxy acetic acid herbicides and phenylurea herbicides, organophosphorus pesticides, and vinclozolin fungicides have been introduced.
To demonstrate the use of immunoaffinity sample preparation, a sample cleanup of coconut waste for the analysis of aflatoxins using the Venture AF Immunoaffinity column was performed. Aflatoxins are high toxic natural substances and suspected carcinogens produced by mold that can be found on agricultural products such as grains, corn, peanuts, and seeds. There are four aflatoxins that are commonly analyzed: B1, B2, G1, and G2. The common analysis procedure is to perform LLE, SPE, or column chromatography followed by HPLC analysis with fluorescence detection. Recently, a more selective straightforward cleanup was introduced using off-line immunoaffinity chromatography (47). Figure 6 depicts on-line immunoaffinity cleanup followed by reversed-phase chromatography and fluorescence detection. The Venture AF column contains an antiaflatoxin antibody immobilized on a wide-pore silica and is selective for the compound class.
For sample preparation, a 25-g sample of coconut fiber was shaken with 5 g of sodium chloride and 100 mL of 80% (v/v) acetonitrile in water. A 1-mL volume of this extract was diluted 10-fold with 9 mL of phosphate buffered saline (PBS) buffer consisting of 0.1 M phosphate plus 0.15 M sodium chloride, pH 7.0. A sample of the extract was injected directly onto the immunoaffinity column resulting in the chromatogram of Figure 6. There are a number of matrix peaks at the beginning of the chromatogram but the aflatoxin compounds are cleanly separated from the interferences. The limit of detection of the method was determined to be 2–4 pg (0.2–0.4 μg/kg depending upon the aflatoxin).
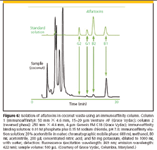
Figure 6
Class- or Ion-Specific SPE Cartridges
Over the years, specialty phases have been introduced that are compound-, class- or element-specific. Of course, the immunoaffinity- and MIP-SPE phases are on the extreme ends of the selectivity scale. Specialty phases have special functional groups that can interact with certain compounds and have found use in niche applications. I would like to discuss briefly a few of these phases that might be of interest.
Immobilized Phenylboronic Acid (PBA) Phases: when the phenylboronic acid SPE phases (Figure 7) are treated with alkaline buffer, they become very specific for certain functional groups such as vicinol diols, which are present in sugars and catechols. Other difunctionalities also can be reactive. For example, α-hydroxy acids, aromatic o -hydroxy acids and amides, and amino alcohol–containing compounds can be retained. They actually form covalent bonds with these groups. When bonded, the sorbents can be washed with any number of solvents to remove interferences. Once washed, the covalent bonds can be broken by washing the phase with an acidic buffer–solvent that hydrolyzes the covalent bonds. A popular application of the PBA phases is the isolation of catecholamines in biological fluids.
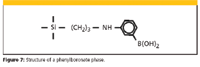
Figure 7
Crown ethers as SPE phases: The unusual ability of the crown ethers to selectively recognize cations has led to the development of unique SPE phases that display excellent selectivity for a class of metal ions or even a single metal ion in the presence of other ions orders of magnitude higher in concentration (48) . In particular, the 3M Rad Disks (St. Paul, Minnesota) combine the Empore PTFE disk technology with the crown ether specificity to provide sorbents that are extremely selective for radium, technetium, and strontium. The Rad Disks have found applications for the selective removal of radiochemicals from aqueous solution. Trace levels of technetium-99 can be determined in the presence of many other ions that might be present in drinking water, surface water, and ground water. Nitrate levels up to 1000 mg/L do not interfere.
2,4-Dintrophenylhydrazine (2,4-DNPH) Phases for Carbonyls: The compound 2,4-DNPH reacts with carbonyl-containing compounds to form a hydrazone. This reaction can occur in solution but is more convenient in a solid state when the 2,4-DNPH is loaded onto an SPE support such as silica gel. For the analysis of aldehydes and ketones in air, the air is passed through the loaded cartridge where the orange hydrazone is formed. After a suitable collecting time, the hydrazone is eluted with organic solvent and the derivatives are analyzed by reversed-phase HPLC. U.S. Environmental Protection Method TO-11A and the American Society for Testing Materials (ASTM) Method D5197-03 specify how the experiment is performed. Several prepackaged SPE cartridge and disk products are available for this application.
Ion removal by SPE: in many applications, especially in ion chromatography, high concentrations of ionic components from the sample matrix can be undesirable interferences. The use of ion-exchanger resins with specific functionalities can be used to remove these ionic species. For example, a strong cation exchanger in the barium form will selectively remove high concentrations of sulfate from aqueous solution. The same can be said for a cation ion exchanger in the silver for the removal of chloride ion. Chelating ion exchangers can remove transition metals.
For example, salt brine contains large amounts of sodium chloride and if one is interested is the analysis of trace amounts of other anions, chloride ion can dominate the chromatogram. Figure 8 shows an example of how an Extract-Clean SPE IC-Ag cartridge (Grace Alltech, Columbia, Maryland) can help in this situation. The left chromatogram shows how chloride dominates the ion chromatogram while after removal of the chloride ion a much cleaner chromatograms is observed.
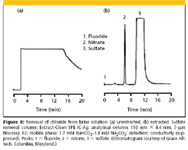
Figure 8
Conclusion
Although conventional SPE phases still dominate the sample preparation world, there are other technologies that can solve problems that might be difficult to achieve with conventional phases. Highly selective phases such as immunoaffinity and MIPs can aid in situations where the conventional phases do not provide the extraction efficiency. On the other hand, there is a limited number of each type of these selective phases, so unless you happen to have analytes that fit into one these categories, you are probably out of luck. If you have a particularly difficult analyte and conventional approaches have been unable to develop a suitable SPE method, having a custom-made MIP synthesized could be an option. The RAM phases, after early startup problems, have proven to solve problems in the analysis of drugs in biological fluids, but one must use them with a column switching setup for best effectiveness. Lifetimes of RAMs have increased in these applications. The multimodal and mixed phases can be useful for selective extractions because they use multiple separation mechanisms (for example, reversed-phase and cation exchange). There are a number of selective phases for compound classes that may be optimum for your particular analytes. There are more than 30 companies with SPE products, so a quick perusal of their websites can give a good overview of what is available.
Ronald E. Majors "Sample Prep Perspectives" Editor Ronald E. Majors is a Senior Chemist, Columns and Supplies Division, Life Sciences and Chemical Analysis Group, Agilent Technologies, Wilmington, DE, and is a member of LCGC's editorial advisory board. Direct correspondence about this column to "Sample Prep Perspectives," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgc-mag.com
Erratum
In the November installment of "Column Watch" (Top 10 HPLC Column Myths; LCGC 24[11], 1172 [2006]), reference 4 is missing from the reference list. It should be
(4) R.E. Majors, LCGC 21(12), 1124–1133 (2003).
References
(1) B.A. Bidlingmeyer, LCGC2(8), 578–580 (1984).
(2) R.E. Majors, LCGC 4(10), 972–984 (1986).
(3) C. Markell, D.F. Hagen, and V.A.Bunnelle, LCGC 9(5), 332–337 (1991).
(4) C.L. Arthur, D.W. Potter, K.D. Buchholz, S. Motlagh, and J. Pawliszyn, LCGC 10(9), 656–661 (1992).
(5) D.D. Blevins and S.K. Schultheis, LCGC 12(1), 12–16 (1994).
(6) R.E. Majors, LCGC 19(7), 678–687 (2001).
(7) K. Ensing, C. Berggren, and R.E. Majors, LCGC 19(9), 942–954 (2001).
(8) W.J. Hurst, LCGC 6(3), 216–218 (1987).
(9) P.R. Locanto, LCGC 9(7), 460–465 (1991).
(10) P.R. Locanto, LCGC 9(11), 752–760 (1991).
(11) H. Wang, W. Liu, and Y. Guan LCGC 22(1), 16–24 (2004).
(12) E.S.P. Bouvier, LCGC 13(11), 852–858 (1995).
(13) R.E. Majors, LCGC 11(5), 336–342 (1993).
(14) L. Jordan, LCGC 11(9), 634–638 (1993).
(15) D.A. Wells, LCGC 17(7), 600–610 (1999).
(16) D.A. Wells, LCGC 17(9), 808–822 (1999).
(17) J. Horack and R.E. Majors, LCGC 11(2), 74–90 (1992).
(18) R.E. Majors and D.E. Raynie, LCGC 15(12), 1106–1117 (1997).
(19) R.E. Majors, LCGC 16S, 3–21 (1998).
(20) E.J. Rook, M.J.X. Hillebrand, H. Rosing, J.M. van Ree, and J.H. Beijnen, J. Chromatogr. B 824, 213–221 (2005).
(21) A. Koole , J. Bosman, J.P. Franke, and R.A. de Zeeuw, J. Chromatogr. B, 726, 149–156, (1999).
(22) M. Raisglid and M.F. Burke, "Solid Phase Extraction Using Layered, Mixed, and Single Sorbent Phases," Pittsburgh Conference, Abstract No. 653, Atlanta, Georgia, 1997.
(23) K.-S. Boos and A. Rudolphi, LCGC 15(7), 602–611 (1997).
(24) K.-S. Boos and A. Rudolphi, LCGC 15(9), 814–823 (1997).
(25) N.M. Cassiano, V.V. Lima, R.V. Oliveira, A.C. de Pietra, and Q.B. Cass, Anal. Bioanal. Chem. 384(7–8), 1462–1469 (2006).
(26) D.R. Doerge, M.L. Churchwell, and K. Berry Delclos, Rapid Commun. in Mass Spectrom. 14, 673–678 (2000).
(27) Y.-C. Chang, C.-M. Li, L.-A Li, S.-B. Jong, P.-C. Liao, and L.W. Chang, Analyst 128, 363–368 (2003).
(28) K. Ensing, C. Berggren, and R.E. Majors, LCGC 19(9), 942–954 (2001).
(29) S.G. Dmitrienko, V.V. Irkha, A. Yu. Kuznetsova, and Yu. A. Zolotov, J. Anal. Chem. 59(9), 808–817 (2005).
(30) C. Baggiani, L. Anfossi, and C. Giovannoli, Current Pharma. Anal. 2(3), 219–247 (2006).
(31) J.O. Mahony, K. Nolan, M.R. Smyth, and B. Mizaikoff, Anal. Chim. Acta 534, 31–39 (2005).
(32) P.A.G. Cormack and A.Z. Elorza, J. Chromatogr. B 804, 173–182 (2004).
(33) Sergey Piletsky and Anthony Turner, Molecular Imprinting of Polymers (Landes Bioscience, Austin, Texas, 2006), p. 220.
(34) C.I. Lin, A.K. Joseph, C.K. Chang, Y.C. Wang, and Y.D. Lee, Anal. Chim. Acta 481, 175–180 (2003).
(35) C. Schirmer and H. Meisel, J. Chromatogr. A 1132, 325–328 (2006).
(36) L. Andersson, E. Hardenberg, M. Sandberg-Stall, K. Moller, J. Henriksson, I. Bramsby-Sjostrom, L.-I.Olsson, and M. Abel-Rehim, Anal. Chim. Acta 526, 147–154 (2004).
(37) P. Martin, I. D. Wilson, D.E. Morgan, G.R. Jones, and K. Jones, Anal. Communications 34, 45–47 (1997).
(38) F. Chapuis, V. Pichon, F. Lanza, B. Sellergren, and M.-C. Hennion, J. Chromatogr. B 804, 93–101 (2004).
(39) R. Curti and U. Colombo, J. Am. Chem. Soc. 74(15), 3961 (1952).
(40) J. Haginaka, Chromatography 23(1), 8 (2002).
(41) B. Sellergren, J. Chromatogr. 906 (1–2), 227–252 (2001).
(42) M.-C. Hennion and V. Pichon, J. Chromatogr. A 1000, 29–52 (2003).
(43) V. Pichon, N. Delaunary-Bertoncini, and M.-C. Hennion, in Sampling and Sample Preparation for Field and Laboratory (Comprehensive Analytical Chemistry), J. Pawliszyn, Ed. (Elsevier Science, Amsterdam, 2002), pp. 1081–1100.
(44) N. Delaunay, V.Pichon, and M.-C. Hennion, J. Chromatogr. B 745, 15 (2000).
(45) D. Stevenson, J. Chromatogr. B 745, 39 (2000).
(46) N. Delaunay-Bertoncini, V. Pichon, M.-C. Hennion, Chromatographia 53, S224 (2001).
(47) J. Stoker et al., J.A.O.A.C. 83(2) 320–340 (2000).
(48) J.S. Bradshaw and R.M. Izatt, Accounts of Chem. Res. 30, 338–345 (1997).

Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.







