The Expanding Role of LC–ICP-MS Speciation Analysis in the Life Sciences: Element- Selective Screening and Species-Independent Quantification
LCGC North America
Emerging as a complementary analytical tool to LC-MS, LC-ICP-MS brings added value to the determination of inorganic element containing molecules of biological importance.
Proper life functionality critically depends upon a number of trace elements that play key roles in natural biochemical processes. However, some other elements are toxic, mutagenic, or carcinogenic. It has become clear that toxicological, biological, and pharmaceutical properties of a particular element often depend upon the species in which the element occurs. Therefore, in a biological sample, the analytical information on the element-containing species, rather than that on the total element content, is of significant importance to answer the questions raised in many current studies of bioinorganic molecules in the life sciences. Those bioinorganic molecules include small organoarsenic and organoselenium compounds, metal-containing drugs and their metabolites, and macromolecular compounds that contain an inorganic element (P, S, Br, I, Si, Se, As, and metals), such as metalloproteins and sulfur-containing peptides.

Speciation: From Inorganic Ions to Proteins
Elemental speciation, defined as the analyses that lead to determining the distribution of an element's particular chemical form in a sample (1), traditionally refers to the determination of elemental species according to the valence states or low molecular weight organic molecule binding to the element of interest using a separation module and an elemental-specific detector. With the rapid development of modern proteomics, the scope of speciation has been extended to the investigation of metal, metalloid, or other inorganic element-containing proteins and peptides. Transition metals and some other inorganic elements, such as P, S, and Se, are common components of cellular proteins. In living organisms, more than a third of cellular proteins have a functional requirement for at least one catalytic or structural metal ion cofactor (2). However, the mechanisms by which the metal is sensed, stored, or incorporated as a cofactor in a cell are rarely known (3). Thus, the identification and characterization of the members of these different classes of metalloproteins recently has become a new subject of investigation in life sciences and is referred to as metalloproteomics. The fact that many known drug targets, such as polymerases, protein kinases, and proteases, are absolutely dependent upon metal cofactors further drives the emergence of "metallomics," which is termed as the complete characterization of metalloproteins, metalloenzymes, and other metal-containing biomolecules that are defined as "metallomes" in a cell or tissue (4). All of these recent advances in life sciences necessitate the existence of viable analytical techniques to identify and quantify those metal complexes.
LC–MS and LC–ICP-MS for Speciation Analysis
The combination of liquid chromatography (LC) with mass spectrometry (MS), in particular atmospheric pressure ionization (API) MS, has been a popular tool for speciation analysis in many bioresearch laboratories because it is readily available as an indispensable structure elucidation technique. Despite its popularity and capability, MS does not seem able to provide reliable analyses of many bioinorganic molecules at trace levels, mainly because of its poor sensitivity for many elements. This poor sensitivity results from the dramatic loss of ionization efficiency in the presence of salt-rich biomatrices (ion suppression). Additionally, interferences from complex matrices also inhibit the ability to measure trace levels.
However, inductively coupled plasma mass spectrometry (ICP-MS), which also is termed as elemental MS and is separated historically from organic MS, is employed widely as an ultrasensitive technique for total trace element analysis and for elemental speciation if combined with a separation module, such as LC and capillary electrophoresis (CE). Except for the main structural elements of organic molecules (that is, C, H, O, and N), ICP-MS can detect many other elements of the biosphere, including P, S, Br, I, Si, Se, As, and all metals. In addition, ICP-MS is able to measure most elements at the part-per-billion level or lower. Because the typical ICP-MS sample introduction flow rate is compatible with most LC separations, coupling ICP-MS to an LC system is very simple and straightforward: directly connect the LC column outlet to the nebulizer of ICP-MS. However, it should be noted here that the compatibility of LC mobile phase composition is a particularly important consideration when ICP-MS is coupled to LC. Aqueous solutions and mobile phases of low organic content can be introduced directly to ICP-MS; however, the presence of high concentrations of organic solvents can result in an unstable plasma, carbon build-up on the interface cones, and ultimately can extinguish the plasma (5). In addition, nonvolatile buffer salts also can deposit on the cones, which can result in signal drift and the need to frequently clean the cones. If a mobile phase of high organic content must be employed for the LC separation, several approaches can be used to make the analysis more robust: desolvating the organic solvent before it reaches the plasma, addition of oxygen between the spray chamber and ICP torch, increasing the radio frequency power applied to the plasma, cooling the spray chamber to condense the solvent vapor, or using postcolumn solvent splitting. Additionally, a narrow-bore column can be used for the separation because this type of column requires a lower flow rate. Because changes in solvent composition alter the plasma properties, gradient LC separations should be monitored carefully.
When combined with an LC, ICP-MS can be used as either an element-specific or a species-specific detector. Because of the ultralow detection limits of ICP-MS, nearly matrix- and interference-free operation, large linear dynamic range, and isotope dilution capability, LC–ICP-MS has revolutionized the field of elemental speciation, in particular for small sized metal binding molecules. Numerous publications have become available about this technique in the last decade. Although the benefits of LC–ICP-MS have been appreciated widely by the elemental speciation community, its application in the life sciences is still much smaller than LC–MS. A wider acceptance might be hindered by the fact that ICP-MS cannot identify the structure of a molecule. As a result, species identification can be accomplished only by matching the retention times of species in the sample to standards.
Recent years have seen growing popularity of LC–ICP-MS in the life sciences as an attractive supplementary technique to molecular MS for the detection and identification of metal-containing biomolecules in complex biological matrices. The ability of ICP-MS to selectively determine other biologically important elements, such as P, S, and Se, makes the technique even more attractive. As a result, the number of biological applications showing the two techniques used in a synergistic way with the aim of an analytical result with molecular specificity has been growing rapidly. The motivation behind this growth could be the continuing search for a capable detection method to solve the problems that MS alone is unable to handle. In most of published applications, however, the recognized advantages of ICP-MS still are limited to better sensitivity, selectivity, and less matrix interference than MS, and quantitative analyses are still based upon the same strategy as MS, which requires species-dependent calibration: pure standard reference for each compound. The purpose of this article is to provide insights into the capabilities that LC–ICP-MS can offer to expand its application in the life sciences, with emphasis on two unique potentials that can be used to complement corresponding LC-MS analysis: elemental selective screening and species-independent calibration. Neither of these has been investigated thoroughly and appreciated. Contrary to species-dependent calibration, species-independent calibration here refers to using a single standard as generic reference to quantify a whole class of analytes. Because this article only summarizes a limited number of publications, interested readers are encouraged to consult recent publications for comprehensive reviews of LC–ICP-MS applications in the life sciences, as well as the technical considerations of interfacing an ICP-MS to an LC system (6,7).
Element-Selective Screening
Preliminary screening of a biological sample is an important, informative step in the initial characterization of the numerous unknown and unclassified biomolecules coming through the compound pipeline. Due to the rich structural information offered by MS and MS-MS, full-scan LC–MS has been used extensively for the analysis of biological samples to identify metabolites, impurities and novel bioactive compounds. However, this is a difficult and time-consuming process. Because of the data-intensive nature of full-scan MS, considerable time and effort is required to extract and interrogate data needed to identify unknowns and expected compounds from the numerous components contained in a complex biological sample. Often, some compounds are not detected by full-scan MS, sometimes because their signals are buried in the spectral noise, and sometimes because poor ionization for metal-binding molecules occurs. Even with the help of searchable MS (or MS-MS) spectral databases, it is an extremely difficult task for analysts.
Differing from atmospheric pressure ionization, the ICP ionization source is an argon plasma that operates at temperatures of 8000 K. In this environment, elements are atomized and ionized into positive charged ions that are transferred selectively to the mass analyzer for determination according to their mass-to-charge ratios. Because of the high-temperature plasma, matrix and analyte molecules are all broken down into individual atoms before ionization. The ionization efficiency of individual element is, theoretically, free of matrix interference and independent of the elemental species. Thus, the ICP-MS response is correlated directly to the absolute amount of the element, and the structure of the compound has no significant influence on ionization efficiency.
LC–ICP-MS produces much clearer chromatograms, with fewer interferences and components, and less signal noise compared to that of a corresponding LC–MS analysis in a total ion (full) scan mode (Figure 1) (8). As shown in Figure 1a, under typical LC–MS conditions, the presence of remarkable background noise makes it almost impossible to distinguish the target peaks. When LC–ICP-MS is employed, the target peaks are clearly visible because of the dramatic reduction in background noise and interferences (Figure 1c). Because only the analytes containing the selected ICP-MS detectable element will be "seen," LC–ICP-MS could be a valuable tool to screen and preidentify a class of biomolecules containing one or more ICP-MS detectable elements in a sample. For example, high-throughput metal screening of proteins can be used to investigate the properties of proteins and place them within the context of the appropriate cellular pathway (9). The identification of one or more heavy metal ions in genomic targets provides opportunities for rapid structural analysis of proteins (10).

Figure 1
A few other applications have utilized the benefit of the highly selective elemental screening capability of LC–ICP-MS to complement MS analysis. Lobinski and colleagues (11) analyzed selenocompounds in nutritional yeast supplements by both LC–ICP-MS and LC–electrospray (ES)–MS. While only four selenium-containing fractions were identified by LC–ES–MS, five were detected by LC–ICP-MS analysis. The undetected peak by LC–ES–MS was due to the ES signal suppression by matrix salt. Even for the detectable species, the ES–MS signals for selenocompounds were difficult to distinguish from the noise. With the help of LC–ICP-MS, a previously unreported selenocompound, whose structure was identified with a follow-up LC–ES–MS analysis, was detected from a complex matrix.
Conventional methods for studying the metabolic fate of I-, Cl-, or S-containing drugs generally require the use of radiolabeling for the tracking and detecting metabolites in complex biofluids by molecular MS. Studies performed without a suitable tracer always have the possibility that important metabolites might be missed, especially if their structures vary significantly from the parent. Wilson and colleagues (12) reported the use of high performance liquid chromatography (HPLC)–ICP-MS to selectively detect compounds containing 35 Cl, 37 Cl, and 32 S, followed by MS identification of phase I and II urinary metabolites of diclofenac, a chlorine-containing drug for rheumatic disease. Without the need for radiolabeled drug standards, different kinds of metabolites were determined in this way, including a previously unreported in vivo metabolite. LC–ICP-MS also has been used to track low concentration drug impurities in the preparation of drugs containing ICP-MS detectable elements (13).
In summary, these studies demonstrated that molecular MS alone seems to be insufficient to allow a comprehensive detection of compounds that has never been reported before, and that LC–ICP-MS is a valuable tool to prespot compounds containing an ICP-MS detectable element that could be invisible to LC–MS analysis.
Species-Independent Quantification
Understanding of the precise mechanism of bioprocesses requires not only the identity but also, often more importantly, the quantity of the biomolecules involved. Quantitative determination of trace or ultratrace species in biological samples remains a very difficult task for the analysts because sample amounts are limited, and reference materials and even simple, pure standards are not always readily available (or are extremely expensive) for most biomolecules of natural origin or metabolites of synthesized drugs. Commonly used species-specific LC detectors, such as UV and MS, measure species dependent signal and require pure standards of each analyte to set up calibrations relating the detector response to the concentration of the analyte. In regulated environments, quantitative measurements must be validated using certified reference materials. Without pure standards, the quantification of those components by a species-specific detector seems impossible. Therefore, to date, speciation studies in life sciences have been aimed at obtaining qualitative (detection and identification) rather than quantitative (determining how much is present) information.
The most promising area of LC–ICP-MS application in life sciences is emerging from here. As discussed previously, the ICP-MS signal only depends upon the absolute amount of the element of interest and the influence of the chemical structures of individual analysts on the signal intensity can be neglected. This creates a sound basis for a species-independent quantification strategy: the quantification of all the biomolecules in a class can be conducted by quantifying an ICP-MS detectable element that defines the class instead of measuring the biomolecules themselves. If variations coming from the LC part are not significant enough to affect the accuracy, a single standard can be used as a generic reference for the whole class of analytes. In some cases where a generic response for all the species is not achievable for accurate quantification, the semiquantitative results still have the benefit of helping to draw a rough conclusion of the analytes' concentrations in the sample. As discussed earlier, the ICP-MS detectable elements comprise all metals and many elements of biological importance such as P, S, Br, I, Si, Se, and As. The element used for quantification can be a naturally occurring element in the biomolecules or, more promisingly, can be an element intentionally introduced as a tag by means of derivatization (ICP-MS tag). The latter concept opens a door of new analytical options and strategies to quantify many biomolecules that cannot be quantified accurately by LC–MS because of the lack of pure standards. The theory and mathematics behind this strategy are very simple: the concentration of the species can be calculated based upon the stoichiometry (element-to-molecule ratio) if the identity of the species is known.
For example, two popular amino acids, cysteine (C3H7NO2S) and methionine (C5H11NO2S), have molecular weights of 121 and 149, respectively, and the weight fraction of S (MW = 32) in those two molecules is 0.264 and 0.215, respectively. Given that the S concentration is found to be 1 mg/L for both compounds separated by LC, the concentrations of cysteine and methionine can be calculated easily to be 3.78 mg/L and 4.67 mg/L (concentration of S/S weight fraction), respectively. Statistically, peptides with a length of 20 or more amino acids contain at least one methionine or cysteine, the quantification of methionine or cysteine eventually will lead to the quantification of the target peptides and proteins.
The potential quantification strategy for biomolecules was realized by several groups, although the benefit has not been exploited fully in literature. Since 2000, Jones and colleagues (14) conducted research demonstrating the use of a single carbon calibration to quantify several amino acids by LC–ICP–atomic emission spectroscopy (AES). They found the responses of the analytes to the carbon emission detector are correlated directly to the mass of carbon in the analytes, and structures of the analyte have no significant affect on the quantification. The following studies conducted in this laboratory were aimed at using LC–MS and LC–ICP–AES in parallel to determine platinum-containing species and their DNA adducts. The LC–MS system was responsible for the identification of these platinum-containing species, while the LC–ICP system quantified these species against a calibration constructed by a readily available standard (15). The LC–ICP-MS system successfully quantified several platinum-containing compounds and their Pt-DNA adducts without the availability of pure standards of those complexes. Axelsson and colleagues (16) investigated the validity of using LC–ICP-MS to provide a genetic detection for structurally noncorrelated compounds with elements like phosphorus and iodine, including phosphatidyl-glycero, monophosphate-peptide, and diphosphate-peptide. The authors found that the response of those compounds to ICP-MS was generic and independent of the structure (Table I), and the detection of selected elements by ICP-MS gives a much more accurate quantification of tested "unknowns" than organic MS detection when the same compounds are not used as calibrants. The application of LC–ICP-MS to phospholipid determination showed that ICP-MS can be used as a detector for the quantification of different phospholipids with the aid of only a single calibration curve, obtained using any phospholipid available.
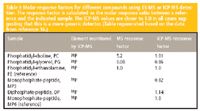
Table I
Quantitative analysis of a new, real-world compound with no pure standard available was conducted by LC–ICP-MS in a study of dimethylarsinoyl compounds in marine samples. The concentration of a novel arsenic compound, dimethylarsinoyl propionate (DMAP), along with dimethylarsinoyl acetate (DMAA) and dimethylarsinoyl ethanol (DMAE), was determined using cation-exchange LC–ICP-MS against an external DMAA calibration curve (17). The new compound was first detected by an LC–ICP-MS arsenic screening in these marine samples. Because its retention time did not correspond to any of the arsenic standards available, the fraction containing the unknown peak was collected manually, and the peak was identified as a novel compound by subsequent ES–MS-MS analysis.
Peptides and proteins are the most intensively studied biomolecules in the life sciences. Lehmann and colleagues (18) developed a method for phosphopeptide identification by capillary LC–ICP-MS, in which ICP-MS is used for 31 P detection. They concluded that using LC–ICP-MS with 31 P detection for phosphopeptide identification is highly selective, and that the signal intensity is independent of the chemical form of phosphorus and directly proportional to the molar amount of 31 P in the LC eluent. Later, the quantification of phosphoproteins, phosphopeptides, and other proteins was accomplished by using ICP-MS to determine phosphorus or sulfur content in the analytes (19,20). The integral peak areas of the 32 S ICP-MS signal showed an average value of 2.8 per sulfur atom in different sulfur-containing peptides, with a maximum deviation of 14% (19). The simultaneous determination of 31 P and 32 S was believed to be a useful tool for the determination of the phosphorylation degree of phosphoproteins (20). In those studies, only an internal standard was used.
Based upon these preliminary findings, we have reason to believe that LC–ICP-MS has the capability to accurately quantify all the compounds incorporating an ICP-MS detectable element by using a single calibration constructed by any readily available standard. The use of an ICP-MS detectable element, naturally present in a biomolecule or introduced as an ICP-MS tag, represents an attractive and promising prespotting and quantification method for peptide, proteins, and drug metabolites.
Conclusions and Expectations
Though the number of publications in LC–ICP-MS speciation is increasing steadily, this technique is still considered an immature tool and has not been applied widely to the life sciences. Another limitation of LC–ICP-MS is the lack of software to work effectively with this technique for routine or industrial-scale operations. Until recently, there had not been a commercialized software package specifically developed for automating LC–ICP-MS instrumentation. The appearance of the fully integrated software, developed by the manufacturers, can be expected to play a key role in bringing the LC–ICP-MS technique into routine use.
The joint application of element and molecular MS for specific detection and molecular characterization of biomolecules has been the most rapidly growing application area of LC–ICP-MS in the life sciences. In such a way, information on molecular structure, element composition, and quantity of an analyte can be assessed to greater depth and with more confidence than that which is possible with molecular MS alone. The parallel coupling of both MS and ICP-MS to an LC system in an online manner will become the ultimate speciation machine (Figure 2). More promisingly, the species-independent quantification capability will expand the application of LC–ICP-MS in the life sciences, and eventually expand the scope of speciation: analyses which lead to the quantification of biomolecules by introducing ICP-MS elemental tags.
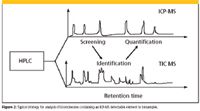
Figure 2
Zheng Yang* and Kenneth Neubauer
Public Health Laboratory Division, Minnesota Department of Heath, and PerkinElmer Life and Analytical Sciences Please direct correspondence to Zheng Yang at Zheng.Yang@state.mn.us
References
(1) J.A. Caruso, K.L. Sutton, and K.L. Ackley, Elemental Speciation. New Approaches for Trace Element Analysis, Comprehensive Analytical Chemistry, Vol. XXXIII (Elsevier, Amsterdam, The Netherlands, 2000).
(2) A.C. Rosensweig, Chem. Biol. 9, 673–677 (2002).
(3) R.J.P. Williams, Coord. Chem. Rev. 583, 216–217 (2001).
(4) H. Haraguchi, J. Anal. At. Spectrom. 19, 5–14 (2004).
(5) H.E. Taylor, R.A. Huff, and A. Montaser, Novel applications of ICPMS in Inductively Coupled Plasma Mass Spectrometry, A. Montaser, Ed. (Wiley-VCH, New York, 1998).
(6) M. Wind and W.D. Lehmann, J. Anal. At. Spectrom. 19, 20–25 (2004).
(7) J. Szpunar, Analyst 130, 442–465 (2005).
(8) B.P. Jensen, C.J. Smith, C. Bailey, C. Rodgers, I.D. Wilson, and J.K. Nicholson, J. Chromatogr., B 809, 279–285 (2004).
(9) E. Phizicky, P.I. Bastiaens, H. Zhu, M. Snyder, and S. Fields, Nature 422, 208–215 (2003).
(10) A. Sali, R. Glaeser, T. Earnest, and W. Baumeister, Nature 422, 216–225(2003).
(11) S. McSheehy, P. Pohl, J. Szpunar, M. Potin-Gautier, and R. Lobinski, J. Anal. At. Spectrom. 16, 68–73(2001).
(12) O. Corcoran, J.K. Nicholson, E.M. Lenz, F. Abou-Shakra, J. Castro-Perez, A.B. Sage, and I.D. Wilson, Rapid Commun. Mass Spectrom. 13, 2377–2384 (2000).
(13) E.H. Evans, J. Wolff, and C. Eckers, Anal. Chem. 73, 4722–4728 (2001).
(14) H.L. Peters, K.E. Levine, and B.T. Jones, Anal. Chem. 73, 453–457 (2001).
(15) Z. Yang, Ph.D. dissertation, 2004.
(16) B.O. Axelsson, M. Jornten-Karlsson, P. Michelsen, and F. Abou-Shakra, Rapid Commun Mass Spectrom. 15, 375–385 (2001).
(17) J.J. Sloth, E.H. Larsen, and K. Julshamn, Rapid Commun. Mass Spectrom. 19, 227–235 (2004).
(18) M. Wind, H. Wesch, R. Kellner, and W.D. Lehmann, Anal. Chem. 77, 1957–1962 (2005).
(19) M. Wind, A. Wegener, A. Eisenmenger, R. Kellner, and W.D. Lehmann, Angew. Chem. Int. Ed. 42, 3425–3427 (2003).
(20) M. Wind, H. Wesch, and W.D. Lehmann, Anal. Chem. 73, 3006–3010 (2001).

Characterizing Polyamides Using Reversed-Phase Liquid Chromatography
May 5th 2025Polyamides can be difficult to characterize, despite their use in various aspects of everyday life. Vrije Universiteit Amsterdam researchers hoped to address this using a reversed-phase liquid chromatography (RPLC)-based approach.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











