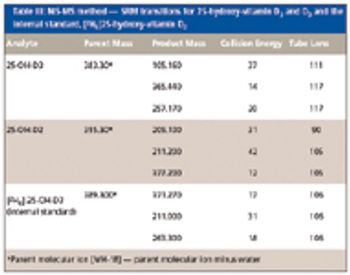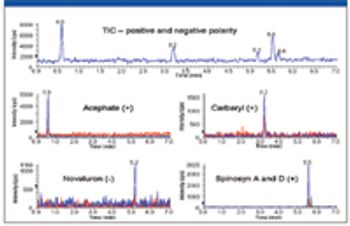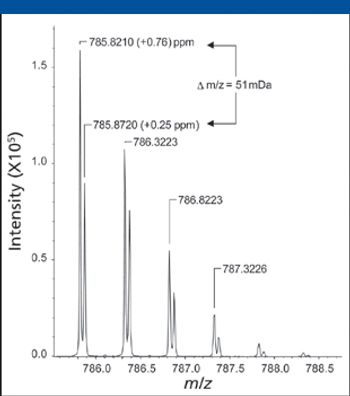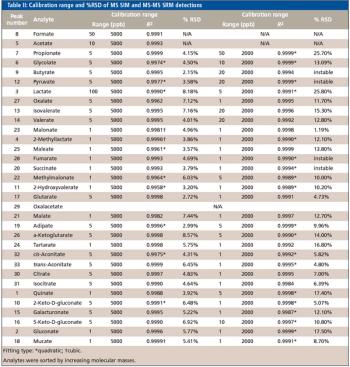
Special Issues
Organic acids are present in many media and play various crucial roles. Low molecular mass organic acids (LMMOAs) have been researched extensively in many areas, such as food chemistry, as these acids affect taste, shelf-life, and food safety (1–3); agriculture, ecosystems, and environmental science, as these acids act as key components in mechanisms that some plants use to cope with environmental stress (4) and fertilizer release (5); biotechnology, to better understand and optimize fermentation processes (6,7); and biomedical research (8–11). Recently, LMMOAs were found to have inhibitory effects on the bioconversion efficiencies of lignocelluloses to ethanol (12).